Effects of Aquatic Physicochemical Parameters Variation on the Phytoplankton Abundance and Diversity in the Babon River, (Semarang, Central Java, Indonesia)
1
Department of Aquatic Resources,
Faculty of Fisheries and Marine Sciences,
Diponegoro University,
Semarang,
Central Java
Indonesia
Corresponding author Email: haeruddindaengmile@lecturer.undip.ac.id
DOI: http://dx.doi.org/10.12944/CWE.19.2.23
Copy the following to cite this article:
Haeruddin H, Purwanti F, Rahman A, Prakoso K. Effects of Aquatic Physicochemical Parameters Variation on the Phytoplankton Abundance and Diversity in the Babon River, (Semarang, Central Java, Indonesia). Curr World Environ 2024;19(2). DOI:http://dx.doi.org/10.12944/CWE.19.2.23
Copy the following to cite this URL:
Haeruddin H, Purwanti F, Rahman A, Prakoso K. Effects of Aquatic Physicochemical Parameters Variation on the Phytoplankton Abundance and Diversity in the Babon River, (Semarang, Central Java, Indonesia). Curr World Environ 2024;19(2).
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2024-05-01 |
|---|---|
| Accepted: | 2024-08-08 |
| Reviewed by: | 
 Chinenye Orji
Chinenye Orji
|
| Second Review by: |

 Dina Hamdy Badawy
Dina Hamdy Badawy
|
| Final Approval by: | Dr. Wilkister Nyaora Moturi |
Introduction
Rivers are essential freshwater ecosystems that support a variety of living organisms, provide habitats for aquatic life, promote tourism, facilitate aquaculture, enable water transportation, serve as waste disposal sites, and act as sources of drinking water.1,2 Increasing water intensively could degrade water quality,3,4 due to contamination of various materials, such as nutrients,5,6, and heavy metals.7-16
Nutrients, especially N and P, can cause a decrease in water quality, eutrophication, and changes in the balance of the natural food webs of aquatic ecosystems.17-19 Meanwhile, metals are toxic, difficult to decompose, and can accumulate in biota tissues.20,21 River water pollution in various parts of the world by nutrients and metals has become a serious concern for multiple groups.22-29
Nutrients in water come from various sources, such as agricultural, industrial, and household activities.22-24,30 Heavy metals in waters come from rock leaching, farming, mining, and industrial activities.31-33 Heavy metals enter the body system of aquatic biota from various pathways34-36
The Babon Watershed is one of the watersheds in Central Java that plays a crucial role in the system's sustainability in the Eastern Semarang region, traversing through the Semarang Regency, Semarang City, and Demak Regency.37 The flow of the Babon River originates from several tributaries from Mount Butak in Ungaran, Semarang Regency. The Babon watershed consists of three sub-watersheds, namely the Gung sub-watershed (8,371.97 Ha), the Pengkol sub-watershed (7,009.65 Ha), and the Babon downstream sub-watershed (9,201.76 Ha) with the main river length of 33.76 km.38 Due to its function as a drainage channel for Semarang City, the Babon River receives a lot of waste input from surrounding activities such as agriculture, industry, and households.39
The composition and population dynamics of plankton can serve as a reliable instrument for biomonitoring studies to evaluate the water quality of aquatic bodies.40 A minor change in physicochemical parameters can influence primary production.41 The temperature and nutrient supply are key environmental factors that influence phytoplankton growth and productivity.42-44
The concentration of CO2 and other gases in the air from transportation, industrial, household, and agricultural activities, causes an increase in air temperature and the occurrence of acid rain, which can increase the temperature and lower the pH of river water. Different changes in phyto and zooplankton potentially resulted from both the effect of acidification due to pH decline and warming due to rising temperatures promoting the growth of phytoplankton over zooplankton.45 This study analyzed the effect of temperature, pH, nutrients, and metals in waters on the composition, dynamics, and structure of phytoplankton communities in the Babon River. So far there is still rarely research conducted to examine the effects of the interaction of temperature, pH, nutrients, and various heavy metals dissolved in water, on the composition, dynamics, and structure of phytoplankton communities in rivers.
Materials and Methods
Research materials include water samples and phytoplankton in the Babon River water. The Horiba Water Checker measures the temperature and pH of river waters in situ. Water samples were collected to analyze nutrient (nitrate and phosphate) and metal (Cd, total Cr, and Pb) concentrations in river water. Phytoplankton samples were collected for phytoplankton composition, abundance, and diversity analysis. Water samples and phytoplankton were collected from 7 observation stations (Figure 1).) representing the upstream (stations 1 and 2), middle (3, 4, and 5), and downstream of the river (6 and 7). The sample collection was carried out during April-May (AM), June-July (JJ), and August-September (AS) 2021. The sample collection was carried out in the morning – afternoon, 1 day each for stations 1–4 and 5–7. Water samples at each station were collected from 3 points (left, center, and right), and then combined into 1 sample for each station.
The collection of phytoplankton was accomplished by filtering 100 liters of water samples were collected using Kemmerer bottles for further storage in sample bottles. All sample bottles were placed in a cooler box during transport to the laboratory to analyze NO3-N, phosphate, Cd, Cr, and Pb using the APHA method.46. The filtered phytoplankton was canal water with a plankton trap mesh size of 35 µm transferred into a sample bottle and fed with a preservative Lugol solution.
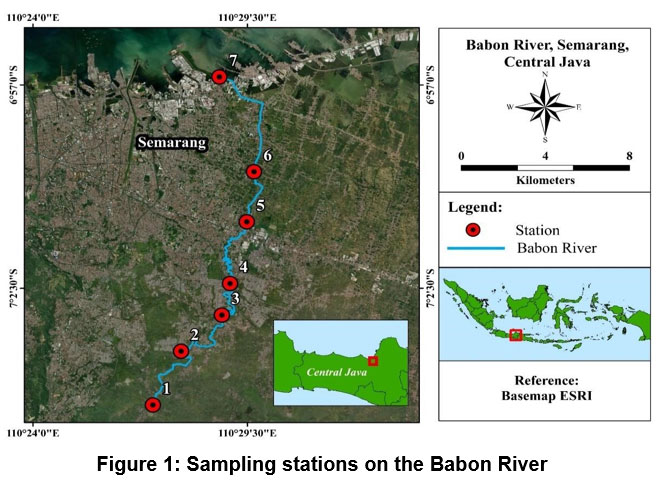 | Figure 1: Sampling stations on the Babon River
|
The analysis of nitrate and phosphate was carried out with a ShimadzuAtomic Absorption Spectrophotometer (AAS)-6200 according to APHA (part 4500NO3) for nitrate,46 and APHA (part 4500-P).46 Analysis of Cd, Total Cr, and Pb was carried out with AAS (APHA, part 3110).46 Phytoplankton were identified through the use of microscopes and reference books. The abundance of phytoplankton was determined using the Sedwick Rafter Count (SRC), while phytoplankton diversity was established using the diversity index.
The collected data is analyzed statistically utilizing suitable software. The data were divided into independent variables (temperature, pH, nitrate, phosphate, Cd, Total Cr, and Pb concentrations) and dependent variables (number of genera, abundance, and phytoplankton diversity index). All variables were analyzed to determine the presence of variations due to the influence of station location, sampling time, and interaction between station location and sampling time. In case the data satisfies the statistical prerequisites for the parametric test, ANOVA is conducted with station location as a factor and sampling time as a block. If the statistical criteria are not met, nonparametric tests are utilized instead. Furthermore, a regression analysis was conducted to fix the contribution of each independent variable to each dependent variable. Previously Principal Component Analysis (PCA) was carried out to simplify a large data set into a smaller set.
Results and Discussion
Results
The data obtained from the measurement of the temperature and pH of river water are presented in Tables 1 and 2. The concentrations of nutrients (nitrate and phosphate) and metals (Cd, Cr, and Pb) are as follows: the lowest and highest nitrate concentrations are 0.125 mg/l and 0.57 mg/l, respectively. The lowest phosphate concentration is 0.008 mg/l, and the highest is 0.383 mg/l (Table 4); While the lowest Cadmium concentration (mg l-1) was 0.0005 and the highest was 0.14 (Table 5); the lowest total Cr concentration (mg l-1) was 0.0005 and the highest was 0.648 (Table 6). The lowest concentration of Lead (mg l-1) was 0.125, and the highest was 0.57 (Table 7).
Table 1: Average water temperature of the Babon River during the study.
Sampling time | Sampling 1 | stations 2 | 3 | 4 | 5 | 6 | 7 |
AM | 27,37 | 29,43 | 29,7 | 28,13 | 31,17 | 30,1 | 28,53 |
JJ | 26,1 | 27,13 | 28,17 | 28,67 | 25 | 29,3 | 29 |
AS | 23,87 | 25,07 | 26,87 | 31,4 | 28,97 | 28,5 | 27 |
Table 2: Average pH of the Babon River During the Study
Sampling time | Sampling stations | 3 | 4 | 5 | 6 | 7 | |
1 | 2 | ||||||
AM | 8.09 | 7.95 | 7.36 | 7.9 | 7.66 | 7.62 | 7.41 |
JJ | 8.29 | 8.07 | 7.89 | 7.99 | 7.45 | 7.42 | 7.31 |
AS | 8.06 | 8.01 | 7.66 | 8.3 | 7.78 | 7.81 | 7.72 |
Table 3: Concentration of nitrate (mg1-l) of the Babon River During the Study
Sampling time | Sampling stations | ||||||
1 | 2 | 3 | 4 | 5 | 6 | 7 | |
AM | 4.120 | 5.01 | 4.85 | 5.23 | 5.25 | 5.24 | 5.41 |
JJ | 0.616 | 0.734 | 1.172 | 0.856 | 0.968 | 0.67 | 0.616 |
AS | 12.679 | 1.108 | 2.106 | 1.578 | 0.851 | 0.53 | 0.381 |
Table 4: Phosphate concentration (mg 1-l) of the Babon River During the Study
Sampling time | Sampling stations | ||||||
1 | 2 | 3 | 4 | 5 | 6 | 7 | |
AM | 0.346 | 0.346 | 0.354 | 0.383 | 0.317 | 0.319 | 0.277 |
JJ | 0.025 | 0.026 | 0.027 | 0.026 | 0.029 | 0.026 | 0.026 |
AS | 0.165 | 0.008 | 0.07 | 0.08 | 0.054 | 0.008 | 0.008 |
Table 5: Cadmium concentration (mg l-1) of the Babon River During the Study.
Sampling time | Sampling stations | ||||||
1 | 2 | 3 | 4 | 5 | 6 | 7 | |
AM | 0.0005 | 0.006 | 0.047 | 0.068 | 0.089 | 0.12 | 0.14 |
JJ | 0.0005 | 0.009 | 0.01 | 0.008 | 0.021 | 0.033 | 0.042 |
AS | 0.0005 | 0.0005 | 0.0005 | 0.0005 | 0.0005 | 0.0005 | 0.0005 |
Table 6: Total Cr concentration (mg l-1) of the Babon River During the Study
Sampling time | Sampling stations | ||||||
1 | 2 | 3 | 4 | 5 | 6 | 7 | |
AM | 0.609 | 0.287 | 0.185 | 0.189 | 0.293 | 0.342 | 0.544 |
JJ | 0.0005 | 0.0005 | 0.171 | 0.648 | 0.394 | 0.434 | 0.457 |
AS | 0.0005 | 0.0005 | 0.0005 | 0.0005 | 0.0005 | 0.0005 | 0.0005 |
Table 7: Concentration of Lead (mg l-1) of the Babon River During the study
Sampling time | Sampling stations | ||||||
1 | 2 | 3 | 4 | 5 | 6 | 7 | |
AM | 0.160 | 0.152 | 0.203 | 0.139 | 0.223 | 0.163 | 0.125 |
JJ | 0.392 | 0.334 | 0.316 | 0.329 | 0.464 | 0.325 | 0.305 |
AS | 0.134 | 0.243 | 0.295 | 0.371 | 0.57 | 0.482 | 0.503 |
The normality test conducted on the data temperature, pH, nitrate, phosphate, Cd Total Cr, and Pb concentrations using the Ryan-Joiner/Kolmogorov-Smirnov method showed that all the data were not distributed normally, so the analysis of variance was carried out using Friedman’s method, where the sampling station as a treatment and blocked by sampling time. The study results showed that the temperature, nitrate, phosphate, Cd, Total Cr, and Pb concentrations between the stations weren’t real differences between sampling stations (p > 0.05). And that only pH was a real difference between stations (p < 0.05). However, if the analysis of variance was carried out using Friedman’s method, where the sampling time was a treatment and blocked by the sampling station, different results of the study temperature and pH not a significant difference between sampling time, there were notable variances in nitrate, phosphate, Cd, Cr, and Pb levels across different sampling times.
The dominant class of phytoplankton is the class Bacillariophyceae. The number of families and genera detected in sampling I was 12, sampling II was 15 and 18 respectively, and sampling III was 11 and 18. The types of phytoplankton found during the study are presented in Figures 2, 3, and 4.
.jpg) | Figure 2: The sum of families and genera of phytoplankton are found in sampling I.
|
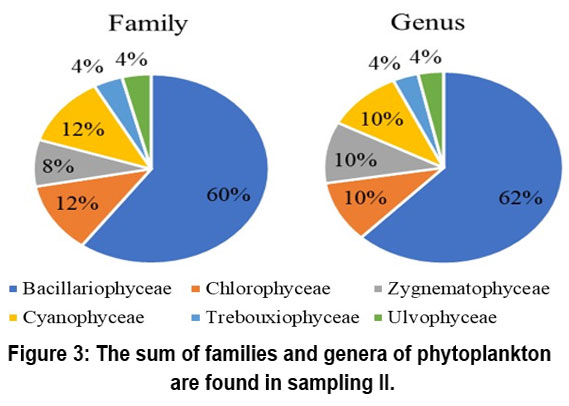 | Figure 3: The sum of families and genera of phytoplankton are found in sampling II.
|
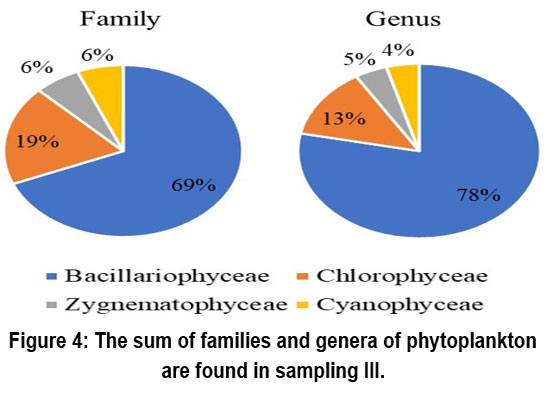 | Figure 4: The sum of families and genera of phytoplankton are found in sampling III.
|
The total count of phytoplankton genera identified across all stations during 3 sampling periods amounted to 30 genera. However, not all genera were discovered at the same time and location during sampling, with some stations only containing 3 genera (lowest) to 16 genera (highest). The lowest number of genera was found at station 4 in sampling 1, while the highest number was at stations 1, 2, 6, and 4 in sampling 2 (Table 8). The lowest phytoplankton abundance is 40 cells/l and the highest is 2545 cells/l (Table 9). The most abundant type of phytoplankton is the genus Navicula, followed by Pediastrum and Nitzchia. The rarest types found are the genera Coscinodiscus, Eunotia, and Rhopadolia. The lowest diversity index was 0.52 and the highest was 2.12.
Table 8: Number of genera of phytoplankton on the Babon River During the study
Sampling time | Sampling Stations | ||||||
1 | 2 | 3 | 4 | 5 | 6 | 7 | |
AM | 9 | 5 | 9 | 3 | 9 | 12 | 6 |
JJ | 16 | 16 | 15 | 13 | 6 | 16 | 13 |
AS | 9 | 5 | 6 | 8 | 10 | 13 | 5 |
Table 9: Abundance (ind. l-1) of phytoplankton on the Babon River During the study
Sampling time | Sampling Stations | ||||||
1 | 2 | 3 | 4 | 5 | 6 | 7 | |
AM | 85 | 40 | 160 | 65 | 245 | 420 | 45 |
JJ | 1805 | 2545 | 1395 | 770 | 155 | 675 | 995 |
AS | 830 | 145 | 180 | 985 | 570 | 1060 | 535 |
Table 10: Phytoplankton Diversity Index on the Babon River During the study
Sampling time | Sampling Stations | ||||||
1 | 2 | 3 | 4 | 5 | 6 | 7 | |
AM | 2.04 | 1.39 | 1.94 | 0.93 | 1.8 | 1.94 | 1.74 |
JJ | 2.12 | 1.99 | 1.95 | 1.91 | 1.37 | 2.12 | 1.84 |
AS | 1.3 | 1.17 | 1.4 | 1.84 | 0.52 | 1.3 | 1.17 |
The results of the Ryan-Joiner/ Kolmogorov-Smirnov Normality Test showed that the number of genera, abundance, and diversity index of all data were not normally distributed, so the variance analysis was carried out using Friedman’s method, in which the sampling station as a treatment and blocked by sampling time. The analysis revealed that there weren’t real differences between sampling stations in ![]() several genera, abundance, or diversity index. Nevertheless, when the analysis of variance was conducted utilizing Friedman's method, with sampling time as the treatment and blocked by the sampling station, it was revealed that there were notable discrepancies in abundance and diversity index across different sampling times, while the number of genera did not exhibit significant differences.
several genera, abundance, or diversity index. Nevertheless, when the analysis of variance was conducted utilizing Friedman's method, with sampling time as the treatment and blocked by the sampling station, it was revealed that there were notable discrepancies in abundance and diversity index across different sampling times, while the number of genera did not exhibit significant differences.
Figure 5 displays a loading plot showcasing the variables that shared the same quadrant: nitrate with phosphate, pH with sampling time, number of genera, phytoplankton abundance with Pb concentration, temperature, Cd, and Cr concentration, and diversity index.
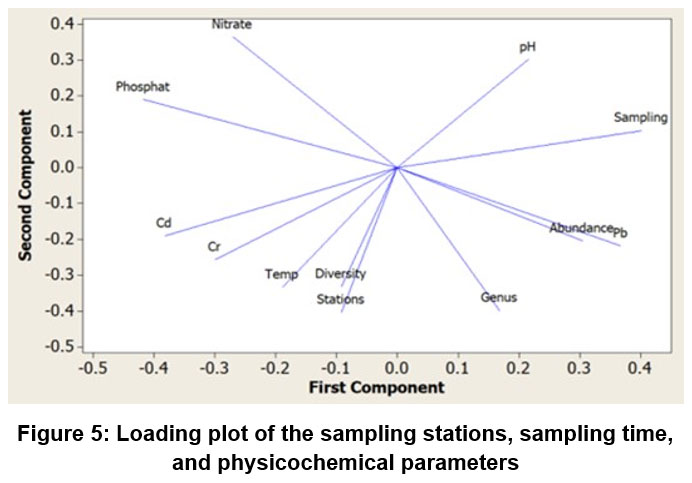 | Figure 5: Loading plot of the sampling stations, sampling time, and physicochemical parameters
|
The pattern of relationships between the independent variables (temperature, pH, concentration: nitrate, phosphate, Cd, Cr, and Pb) and the dependent variables (number of genera, abundance, and phytoplankton diversity index) is as follows:
The pattern of relationships between the independent variables (temperature (X1), pH (X2), concentration: nitrate (X3), phosphate (X4), Cd (X5), Cr (X6), and Pb (X7) and the dependent variables (number of genera (Y1) are: Y1 = - 31.0 + 0.846 X1 + 2.54 X2 + 0.229 X3 - 24.7 X4 + 8.3 X5 + 4.14 X6 - 4.2 X7
The values of the coefficients, SE, T, and P of each variable are as follows:
Predictor | Coef | SE Coef | T | P |
Constant | -30.97 | 37.51 | -0.83 | 0.424 |
Temp | 0.8463 | 0.6267 | 1.35 | 0.200 |
pH | 2.540 | 4.160 | 0.61 | 0.552 |
Nitrate | 0.2292 | 0.5506 | 0.42 | 0.684 |
Phosphate | -24.66 | 12.44 | -1.98 | 0.069 |
Cd | 8.26 | 35.92 | 0.23 | 0.822 |
Cr | 4.143 | 5.220 | 0.79 | 0.442 |
Pb | -4.19 | 13.85 | -0.30 | 0.767 |
The pattern of relationships between the independent variables (temperature (X1), pH (X2), concentration: nitrate (X3), phosphate (X4), Cd (X5), Cr (X6), and Pb (X7) and the dependent variables (abundance and phytoplankton (Y2) are:
The relationship pattern between the independent variables (temperature (X1), pH (X2), concentration: nitrate (X3), phosphate (X4), Cd (X5), Cr (X6) and Pb (X7)) and the dependent variables (abundance and phytoplankton (Y2)) was: Y2 = - 7727 + 64.9 X1 + 923 X2 + 6.6 X3 - 3503 X4 + 3846 X5 - 408 X6 - 649 X7
The coefficients, SE, T, and P for each variable were as follows:
Predictor | Coef | SE Coef | T | P |
Constant | -7727 | 5206 | -1.48 | 0.162 |
Temp | 64.93 | 86.99 | 0.75 | 0.469 |
pH | 922.6 | 577.4 | 1.60 | 0.134 |
Nitrate | 6.65 | 76.43 | 0.09 | 0.932 |
Phosphate | -3503 | 1727 | -2.03 | 0.064 |
Cd | 3846 | 4987 | 0.77 | 0.454 |
Cr | -408.2 | 724.6 | -0.56 | 0.583 |
Pb | -649 | 1923 | -0.34 | 0.741 |
The relationship pattern between the independent variables (temperature (X1), pH (X2), concentration: nitrate (X3), phosphate (X4), Cd (X5), Cr (X6) and Pb (X7)) and the dependent variables (diversity index (Y3)) was: Y3 = - 1.50 + 0.0763 X1 + 0.237 X2 - 0.0292 X3 - 1.76 X4 + 0.38 X5 + 0.643 X6 - 2.37 X7
The coefficients, SE, T, and P values of each variable are:
Predictor | Coef | SE Coef | T | P |
Constant | -1.501 | 3.696 | -0.41 | 0.691 |
Temp | 0.07626 | 0.06176 | 1.23 | 0.239 |
pH | 0.2369 | 0.4100 | 0.58 | 0.573 |
Nitrate | -0.02920 | 0.05427 | -0.54 | 0.600 |
Phosphate | -1.756 | 1.226 | -1.43 | 0.176 |
Cd | 0.383 | 3.541 | 0.11 | 0.915 |
Cr | 0.6429 | 0.5145 | 1.25 | 0.233 |
Pb | -2.368 | -1.365 | -1.73 | 0.107 |
Discussion
Research on the effects of various physic-chemical environmental factors on phytoplankton population dynamics in rivers has been widely conducted.40,41,47-49 The researchers got similar results to the previous researchers' findings, but some are different. Previous studies have demonstrated that water quality parameters, such
However, the influence of the sampling station on the overall research data is more dominant than the sampling time, so all the independent variables studied (temperature, pH, nitrate concentration, phosphate, Cd, Cr, and Pb) did not have a significant effect on the dependent variables (genus number, abundance, and phytoplankton diversity index). Regression analysis to examine the pattern of relationships between the independent variables (temperature, pH, concentration: nitrate, phosphate, Cd, Cr, and Pb) and the dependent variables (number of genera, abundance, and phytoplankton diversity index), showed that all independent variable's observation had no real contribution to the non-observational variables. The abundance of phytoplankton shows a marked difference between sampling times. The difference in phytoplankton abundance indicates that the distribution of phytoplankton is uneven in the waters. Plankton is an organism that has a patchy distribution pattern and a weak ability to move, so its distribution depends on the movement of water masses. Differences in the characteristics of physical and chemical parameters of waters such as fluctuations in currents, temperature, and nutrient concentrations can cause changes in phytoplankton abundance.55 Slow-flowing rivers are more suitable for plankton life.56 Temperature and nutrient concentration can control phytoplankton growth.42-44 The dominant phytoplankton types found are the class Bacillariophyceae, then Chlorophyceae and Cyanophyceae (Appendix 3). This composition is similar to the results of Sharma's research, which found the class Bacillariophyceae dominates 95% of phytoplankton species, followed by Chlorophyceae (2.8%) and Cyanophyceae (1.6%), the rest from other phytoplankton classes.41 Other research by Sharma obtained a similar composition with the dominance by classes Bacillariophyceae (36.3 - 80.5%), Chlorophyceae (18.1– 50.5%), Cyanophyceae (1.1–17.5%), and the rest of other phytoplankton classes.40 Bacillariophyceae class or diatoms are a class of phytoplankton that easily adapt to various environmental conditions, including in polluted waters by multiplying mucus on their body surface.57,58 Chlorophyceae are found in fresh and salt waters. The majority of them occur as submerged, freshwater plants being attached to submerged rocks, wood pieces, and similar other objects, but may also float on the surface of stagnant water forming green scums.59 Cyanophyceae class will dominate waters that are slow-current, nutrient-rich (especially phosphorus), and warm temperatures.60
![]()
Conclusion
The dominant phytoplankton class found in the Babon Rivers during the study consisted of Bacillariophyceae, Chlorophyceae, and Cyanophyceae. The sampling station does not give rise to diversity against all independent variables (temperature, pH, nitrate, phosphate, Cd, Total Cr, Pb concentrations) and dependent variables (number of genera, abundance, and diversity index of phytoplankton). However, the sampling time causes diversity in nitrate, phosphate, Cd, Total Cr, and Pb concentrations. The sampling location effect was more dominant than sampling time, so the results showed that all independent variables did not contribute significantly to the dependent variables.
Acknowledgment
We are grateful to the Dean of the Faculty of Fisheries and Marine Sciences, Diponegoro University, and all his staff for providing a research grant to the first author. Similarly, for all parties who have helped carry out research and article writing, so that this article can be published.
Funding Sources
This research was funded by a research grant from the Faculty of Fisheries and Marine Sciences, Diponegoro University in 2021 Contract number 62/UN7.5.10.2/ PP/2021.
Conflict of Interest
The authors do not have any conflict of interest.
![]()
Data Availability Statement
The research paper includes all statistics information analyzed during the research work.
Ethics Statement
This research did not involve human participants, animal subjects, or any material that requires ethical approval.
Author Contributions
The research work was conducted by Dr. Haeruddin and Dr Frida Purwanti, senior lecturer in the Department of Aquatic Resources, Faculty of Fisheries and Marine Science, Diponegoro University, Central Java, Indonesia.
As young researchers, Arif Rahman and Kukuh Prakoso assisted in the Department of Aquatic Resources, Faculty of Fisheries and Marine Science, Diponegoro University.
Research plans, research contracts, leading data collection in the field, interpreting data, and writing articles are some of the tasks Haeruddin handles.
Frida Purwanti helped develop research strategies, interpret data, and write research papers.
Arif Rahman, M.Sc., prepares materials and tools for data collection, assists in data collection in the field, conducts data analysis, and helps write articles
Kukuh Prakoso, M.Sc., prepares correspondence for data collection permits, prepares transportation, assists in data collection in the field, conducts data analysis, and helps write articles.
References
- Haakonde T., Yabe J., Choongo K., Chongwe G., Islam Md. S. Preliminary Assessment of Uranium Contamination in Drinking Water Sources Near a Uranium Mine in the Siavonga District, Zambia, and Associated Health Risks. Mine Water Environ. 2020; 39: 735–745. https://doi.org/10.1007/s10230-020-00731-5
CrossRef - Islam Md., S. Preliminary Assessment of Trace Elements in Surface and Deep Waters of an Urban River (Korotoa) in Bangladesh and Associated Health Risk. Environ Sci Pollut Res. 2021; 28: 29287–29303. https://doi.org/10.1007/s11356021-12541-5
CrossRef - Anzani, Y. M., Effendi H., Wardiatno Y. Macrozoobenthos as a Bioindicator of Water Quality in the Ciambulawung River, Lebak, Banten. Proceedings of the Annual Scientific Meeting of the Indonesian Limnological Society. 2013. Limnology Research Center. Bogor [Indonesian].
- Çelekli A., Kayhan S., Çetin T. First Assessment of Lakes’ Water Quality in Aras River Catchment (Turkey): Application of Phytoplankton Metrics and Multivariate Approach. Ecological Indicators. 117. 2020; 106706, 1-10. https://doi.org/10.1016/j.ecolind.2020.106706
CrossRef - Moritsch M., Szendrenyi A., Leger C., Frossard B. Associations among Plankton Abundance, Water Quality, and Sediment Quality in the San Francisco Bay: Nitrogen And Phosphorus. Berkeley Scientific Journal. 2011; 14(1): 45-54. http://escholarship.org/uc/item/1zf0t4tr
CrossRef - Sanz-Lazaro C., Fodelianakis S., Guerrero-Meseguer L. A., Marín A., Karakassis I. Effects of Organic Pollution on Biological Communities of Marinebiofilm on Hard Substrata. Environ. Poll. 2015; 201: 17-25. http://dx.doi.org/10. 10.1016/j.envpol.2015.02.032
CrossRef - Aghadadashi V., Neyestani M. R., Mehdinia A., Bakhtiari A. R., Molaei S., Farhangi M., Esmaili M., Marnani H. R., Gerivani H. Spatial Distribution and Vertical Profile of Heavy Metals in Marine Sediments around Iran's Special Economic Energy Zone; Arsenic as an Enriched Contaminant. Mar. Poll. Bull. 2019; 138: 437-450.
CrossRef - Ali M., Ali L., Proshad M. R., Islam S., Rahman Z., Kormoker T. Assessment of Trace Elements in the Demersal Fishes of a Coastal River in Bangladesh: a Public Health Concern. Thalassa. 2020; 36: 641–655.
CrossRef - Cai L-M., Wang Q-S., Luo J., Chen L-G., Zhu R-L., Wang S., Tang C-H. Heavy Metal Contamination and Health Risk Assessment for Children Near a Large CuSmelter in Central China. Sci. Tot. Environ. 2019; 650: 725–733. https://doi.org/10.1016/j.scitotenv.2018.09.081
CrossRef - He Z., Li F., Dominech S., Wen X-H., Yang S. Heavy Metals of Surface Sediments in the Changjiang (Yangtze River) Estuary: Distribution, Speciation and Environmental Risks. Journal of Geochem Explor. 2019; 98: 18-28. https://doi.org/10.1016/j.gexplo.2018.12.015
CrossRef - Hossain Md. S., Ahmed Md. K., Ahmed K., Sarker S., Rahman M. S. Seasonal Variations of Trace Metals from Water and Sediment Samples in the Northern Bay of Bengal. Ecotox. and Environ. Safety. 2020; 193: 110347. http://refhub.elsevier.com/S1001-6279(21)00052-4/sref32
CrossRef - Ilie M., Marinescu F., Szep R., Ghi?a G., Deak G., Anghel A., Petrescu A., Uri?escu B. Ecological Risk Assessment of Heavy Metals in Surface Sediments from the Danube River. Carpathian Journal of Earth and Environ Sci. 2017; 12(2): 437–445.
- Kahal A., Abdelbaset El-Sorogy S., Qaysi S., Almadani S., Kassem O. M., AlDossari A. Contamination and Ecological Risk Assessment of the Red Sea Coastal Sediments, Southwest, Saudi Arabia. Mar. Poll. Bull. 2020; 154: 111125. https://doi.org/10.1016/j.marpolbul.2020.111125
CrossRef - Liu P., Hu W., Tian K., Huang B., Zhao Y., Wang X., Zhou Y., Shi B., Kwon B. O., Choi K., Ryu J., Chen Y., Wang T., Khim J. S. Accumulation and Ecological Risk of Heavy Metals in Soils along the Coastal Areas of the Bohai Sea and the Yellow Sea: a Comparative Study of China and South Korea. Environ. International 2020; 137: 1-12. https://doi.org/10.1016/j.envint.2020.105519
CrossRef - Nour H. E., El-Sorogy A. S. Distribution and Enrichment of Heavy Metals in Sabratha Coastal Sediments, Mediterranean Sea, Libya. Journ. of African Earth Sci. 2017; 134: 222 – 229. http://dx.doi.org/10.1016/ j.jafrearsci.2017.06.019
- Ran X-F., Wu Z-X., Yue H., Fu X-L., Kang Y-H., Xu S., Yang Y-J., Xu J-Z., Shi JQ. The Response and Detoxification Strategies of Three Freshwater Phytoplankton Species, Aphanizomenon flos-aquae, Pediastrum simplex, and Synedra acus to Cadmium. Environ. Scie. and Poll. Research. 2019; 22:19596–19606
- Schindler D. W., Carpenter S. R., Chapra S. C., Hecky R. E., Orihel D. M. Reducing Phosphorus to Curb Lake Eutrophication is a Success. Environ. Sci. Technol. 2016; 50: 8923–8929. https://doi.org/10.1021/acs.est.6b02204
CrossRef - Kosek K., Polkowska ?., ?yszka B., Lipok J. Phytoplankton Communities of Polar Regions Diversity Depending on Environmental Conditions and Chemical Anthropopressure. Journal of Environ. Manag. 26. 2016;171: 27. 243-259. http://dx.doi.org/10.1016/j.jenvman.2016.01.026
CrossRef - Duong T., Hoang T., H., Nguyen T. K., Le T. P. Q., Le N. D., Dang D. K., Lu X., Bui M. H., Trinh Q. H., Dinh T. H. V., Pham T. D., Rochelle-Newallj E. Factors Structuring Phytoplankton Community in a Large Tropical River: Case Study in the Red River (Vietnam). Limnologica. 2019; 76: 82–93. https://doi.org/10.1016/j.limno.2019.04.003
CrossRef - Saha N., Zaman M. R. The concentration of Selected Toxic Metals in Groundwater and Some Cereals Grown in the Shibganj area of Chapai Nawabganj, Rajshahi, Bangladesh. Cur. Sci. 2011; 101(3): 427–431.
- Squadrone S., Brizio P., Stella C., Prearo M., Pastorino P., Serracca L., Ercolini C., Abete M. C. Presence of Trace Metals in Aquaculture Marine Ecosystems of the Northwestern Mediterranean Sea (Italy). Environmental Pollution. 2016; 215: 77- 83. http://dx.doi.org/10.1016/j.envpol.2016.04.096
CrossRef - EEA (European Environmental Agency). Nutrients in Rivers. 2015 .http://www.eea. Europa.EU/sour/2015/countries-comparison/freshwater
- EEA (European Environmental Agency). Nutrition in Fresh Water (CSI 020) - September 2014 Assessment. 2014. https://www.eea.europa.eu/soer/data-andmaps/indicators/nutrients-in-freshwater/nutrients-in-freshwater-assessmentpublished-6
- EEA (European Environmental Agency). European Waters -, EEA Report 2012; No 8/2012, European Environment Agency, Copenhagen. https://www.eea.europa.eu/publications/european-waters-assessment-2012. doi:10.2800/63266Ebrahimpour et al., 2011;
- Mohiuddin K. M., Zakir H. M., Otomo K., Sharmin S., Shikazono N. Geochemical Distribution of Trace Metal Pollutants in Water and Sediments Downstream of a T.R. Choudhury et al. Environ. Nanotech., Monit. & Manag. 202; 16: 100484 Urb. Riv. Int. J. Environ. Sci. Technol. 2010; 7: 17–28. https://doi.org/10.1007/ BF03326113.
CrossRef - Kibria G., Hossain M. M., Mallick D., Lau T. C., Wu R. Monitoring of Metal Pollution in Waterways across Bangladesh and Ecological and Public Health Implications of Pollution. Chemosphere 2016; 165: 1–9. https://doi.org/10.1016/j. chemosphere.2016.08.121
CrossRef - Alengebawy A., Abdelkhalek S. T., Qureshi S. R., Wang M. Q. Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics. 2021; 9(3): 42. https://doi.org/10.3390/ toxics9030042
CrossRef - Jaishankar M., Tseten T., Anbalagan N., Mathew B. B., Beeregowda K. N. Toxicity, mechanism, and health effects of some heavy metals. Interdisciplinary Toxic. 2014; 7(2): 60-72.
CrossRef - Rajeshkumar S., Liu Y., Zhang X., Ravikumar B., Bai G., Li X. Studies on Seasonal Pollution of Heavy Metals in Water, Sediment, Fish, and Oyster from the Meiliang Bay of Taihu Lake in China. Chemosphere. 2018; 191: 626-638. https://doi.org/10.1016/j.chemosphere.2017.10.078
CrossRef - Bhagowati B., Ahamad K. U. A Review on Lake Eutrophication Dynamics and Recent Developments in Lake Modeling. Ecohydrol. Hydrobiol. 2019; 19: 155-166.
CrossRef - Ravindra K., Mor S. Distribution and Health Risk Assessment of Arsenic and Selected Heavy Metals in Groundwater of Chandigarh, India. Environ. Pollut. 2019; 250: 820–830. https://doi.org/10.1016/j.envpol.2019.03.080
CrossRef - Zhao H. J., Wang Y., Yang L., Yuan L. W., Peng D. C. Relationship between Phytoplankton and Environmental Factors in Landscape Water Supplemented with Reclaimed Water. Ecol. Indicators. 2015; 58: 113-121.
CrossRef - Luo M., Yu H., Liu Q., Lan W., Ye Q., Niu Y., Niu Y. Effect of River-Lake Connectivity on Heavy Metal Diffusion and Source Identification of Heavy Metals in the Middle and Lower Reaches of the Yangtze River. J. Hazard. Mater. 2021; 416: 125818. https://doi.org/10.1016/j.jhazmat.2021.125818
CrossRef - Wang D., Lin W., Yang X., Zhai W., Dai M., Chen C. Occurrences of Dissolved Trace Metals (Cu, Cd, and Mn) in the Pearl River Estuary (China), a Large River- Groundwater-Estuary System. Cont. Shelf Res. 2012; 50–51: 54–63. https://doi.org/ 10.1016/j.csr.2012.10.009
CrossRef - Kumar S. B., Padhi R., Mohanty A., Satpathy K. Distribution and Ecological - and Health-Risk Assessment of Heavy Metals in the Seawater of the Southeast Coast of India. Mar. Pollut. Bull. 2020; 161: 111712. https://doi.org/10.1016/j.marpolbul.2020.111712
CrossRef - Li Y., Gao B., Xu D., Peng W., Liu X., Qu X., Zhang M. Hydrodynamic Impact on Trace Metals in Sediments in the Cascade Reservoirs, North China. Sci. Total Environ. 2020; 716: 136914. https://doi.org/10.1016/j.scitotenv.2020.136914.
CrossRef - Prihestiwi R. C., Handayani W., Sarasadi A. Land Use and Surface Runoff Change in Babon Watershed Semarang Greater Area. IOP Conf. Series: .: Earth Environ. Sci. 2020; 2023; 1264: 012020. IOP Publishing. doi:10.1088/17551315/1264/1/012020
CrossRef - Wahyudi R. S., Wahyudi S. I., Subagya P. H. Water Quality Evaluation Used to Function Feasibility Case Study on Babon River in Semarang, Central Java, Indonesia. IOP Conf. Ser.: Earth Environ Sci. 2020; 612: 012034
CrossRef - Haeruddin, Ghofar A., Purwanti F., Rahman A. Final Report of Output-Based Research Grant: Analysis of Assimilation Capacity and Pollution Status of DAS Babon, Central Java. 2021; Faculty of Fisheries and Marine Sciences, Diponegoro University [Indonesian]
- Sharma C., Singh U. B., Jindal R., Ahluwalia A. S. Population dynamics and species diversity of plankton about hydrobiological characteristics of river Sutlej, Punjab, India. Ecol. Environ. Conserv. · 2013; 19(3): 717-724
- Sharma A., Sharma R. C., Anthwal A. Monitoring phytoplankton diversity in the hill stream Chandrabhaga of Garhwal Himalaya. Life Sci. Journal. 2007; 4(1): 80 – 84.
- Eppley R. W. Temperature And Phytoplankton Growth In The Sea. Fish Bull. 1972; 70(4): 1063-1085. https://spo.nmfs.noaa.gov/sites/default/files/pdf-content/ 1972/704/eppley.pdf
- Falkowski P., Oliver M. Mix and match: how climate selects phytoplankton. Nat Rev Microbiol. 2007; 5: 813–819. https://doi.org/10.1038/nrmicro1751
- van de Waal D. B., Litchman E. Multiple global change stressor effects on phytoplankton nutrient acquisition in a future ocean. Phil. Trans. R. Soc. B 375: 20190706. http://dx.doi.org/10.1098/rstb.2019.0706
CrossRef - Wei Y., Cui H., Hu Q., Bai Y., Qu K., Sun J., Cui Z. Eutrophication status assessment in the Taizhou Bay, Bohai Sea: further evidence for the ecosystem degradation. Mar. Pollut. Bull. 2022; 181: 113867
CrossRef - APHA, AWWA, WEF (American Public Health Association, American Water Works Association, Water Environment Federation). Standard Methods for the Examination of Water and Wastewater. 23rd edition. Edited by: Baird R. B., Eaton A.D., and Rice E.W.. APHA, Washington DC. 2017. https://doi.org/10.2105/ SMWW.2882.214
- Hutabarat S., Soedarsono P., Cahyaningtyas I. Plankton analysis study to determine the level of pollution in the estuary of the Semarang Babon River. Journ Manag. Aquat Res. 2013; 2(3): 74-84. http://ejournal-s1.undip.ac.id/ index.php/maquares. [Indonesian]
CrossRef - Kim J. S., Seo I. W, Baek D-H. Seasonally varying effects of environmental factors on phytoplankton abundance in the regulated rivers. Sci Reports. 2019; 9:9266. https://doi.org/10.1038/s41598-019-45621-1.www.nature.com/ scientificreports.
CrossRef - Rahman A., Haeruddin, Ghofar A., Purwanti F. Water Quality Condition and Community Structure of Diatom (Bacillariophyceae) in Babon River. Saintek Perikanan: Indonesian Journ Fish Sci Tech. Available at http://ejournal.undip.ac.id/index.php/saintek. 2022; 18(2):125 - 129. [Indonesian]
CrossRef - Bowes M. J., Gozzard E., Johnson A. C., Scarlett P. M., Roberts C., Read D. S., Armstrong L. K., Harman S. A., Wickham H. D. Spatial and temporal changes in chlorophyll-a concentrations in the River Thames basin, UK: Are phosphorus concentrations beginning to limit phytoplankton biomass? Sci Tot Environ. 2012; 426(1): 45-55.
CrossRef - Song Y, Guo Y, Liu H, Zhang G, Zhang X, Thangaraj S, Sun J (2022) Water quality shifts the dominant phytoplankton group from diatoms to dinoflagellates in the coastal ecosystem of the Bohai Bay. Mar Poll Bull 183:114078. https://doi.org/10.1016/j.marpolbul.2022.114078
CrossRef - Wu N., Guo K., Suren A. M., Riis T. Lake morphological characteristics and climatic factors affect long-term trends of phytoplankton community in the Rotorua Te Arawa lakes, New Zealand during 23 years observation. Water Res 2023; 229:119469. https://doi.org/10.1016/j.watres.2022.119469
CrossRef - Gao W., Xiong F., Lu Y., Xin W., Wang H., Feng G., Kong C., Fang L., Gao X., Chen Y. Water quality and habitat drive phytoplankton taxonomic and functional group. 2 Ecol Process. 2024; 13:11
CrossRef - Semarang City Statistic Central Bureau 2022. Semarang City in 2023 Figures. Statistics Central Bureau. [Indonesian]
- Haumahu S. Phytoplankton spatial distribution in Haria Saparua Bay Waters, Central Maluku. Mar Sci. 2005; 10(3): 126-134. [Indonesian].
- Odum, E.P. Fundamentals of Ecology. 3rd Ed. 1971; 1-574. W.B. Saunders Co., Philadelphia.
- Heramza K., Barour C., Djabourabi A., Khati W., Bouallag C. Environmental parameters and diversity of diatoms in the A?n Dalia dam, Northeast of Algeria. Biodiv. 2021; 22(9): 3633-3644. DOI: 10.13057/biodiv/d220901.
CrossRef - Saxena A., Tiwari A., Kaushik R., Iqbal H. M. N., Parra Saldívar R. Diatoms recovery from wastewater: Overview from an ecological and economic perspective. Journal of Water Proc Eng 2021; 39: 1-14. DOI: 10.1016/ j.jwpe.2020.101705
CrossRef - https://www.botanylibrary.com/algae/greenalgae/Chlorophyceae-or-the-greenalgae-botany/14627
- van Vuuren SJ., Taylor J., van Ginkel C., Annelise Gerber A. Easy identification of the most common freshwater algae. A guide for the identification of microscopic algae in South African freshwaters. North-West University and the Department of Water Affairs and Forestry. 2006.







