Contamination of Water by Heavy Metals and Treatment Methods – A Review
S. K. Suja
*
 , S Almaas
, S Almaas
 , A Prasanna Gracy
, A Prasanna Gracy
 , P Gowsika
, P Gowsika
 , K Jeyapradeepa
, K Jeyapradeepa
 , G Suba Sri
, G Suba Sri
 , S Mathiya
, S Mathiya
 and K Berlin Asha
and K Berlin Asha

1
Department of Chemistry,
Lady Doak College,
Madurai,
Tamil Nadu
India
Corresponding author Email: suja@ldc.edu.in
DOI: http://dx.doi.org/10.12944/CWE.19.1.2
Copy the following to cite this article:
Suja S. K, Almaas S, Gracy A. P, Gowsika P, Jeyapradeepa K, Sri G. S, Mathiya S, Asha K. B. Contamination of Water by Heavy Metals and Treatment Methods – A Review. Curr World Environ 2024;19(1). DOI:http://dx.doi.org/10.12944/CWE.19.1.2
Copy the following to cite this URL:
Suja S. K, Almaas S, Gracy A. P, Gowsika P, Jeyapradeepa K, Sri G. S, Mathiya S, Asha K. B. Contamination of Water by Heavy Metals and Treatment Methods – A Review. Curr World Environ 2024;19(1).
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2023-01-11 |
|---|---|
| Accepted: | 2023-12-27 |
| Reviewed by: | 
 Oiseoje Wangboje
Oiseoje Wangboje
|
| Second Review by: |

 Grigorios Kyriakopoulos
Grigorios Kyriakopoulos
|
| Final Approval by: | Dr. Saravanan Pichiah |
Introduction
Pure water is essential for leading a harmonious life. Due to the vital role of water for humanity, it is very significant to maintain and improve the quality of water. Currently, because of rapid industrialization and technological advancements, the claim for usage of water is high in both the local and industrial regions1. The availability of fresh water is becoming inadequate due to many man-made accomplishments. Because of the growing living standards, the increasing world population surges water supply2. Nowadays the world is facing a great challenge of destruction of the environment particularly by water pollution 2,3,4. The discharge of heavy metals and other toxic pollutants by human and industrial activities has become a major problem for both humans and aquatic life 4. There are some common pollutants like palladium (Pd), Mercury (Hg), Chromium (Cr), Cadmium (Cd) and Nickel (Ni) that are often detected in industrial effluents5,6,7. Heavy metals are important in many aspects to mankind, especially in the manufacturing of accumulators, thermometers, and utensils. If these heavy metals present in higher concentrations beyond the limit, it is hazardous to the lives of both flora and fauna. Due to its bio-toxic effect, it is hazardous to the environment. Many techniques are existing to effectively remove heavy metals from industrial effluents. Some treatments are flocculation, coagulation, adsorption, membrane process, ion-exchange process, chemical precipitation, filtration, solvent extractions, photodegradation and electrodialysis4,5,8. All these techniques have both merits and demerits. In recent days, there are numerous cost efficient and eco-friendly studies available in literature for the elimination of toxic heavy metals from the wastewater and to improve the quality of treated effluent8,9.
Heavy metals
They are trace elements such as Cadmium, Mercury, Arsenic, Nickel, Copper, Lead, Iron, Chromium, Zinc and Cobalt10, 11. Heavy metals are toxic, carcinogenic, non-biodegradable and mutagenic. Heavy metals are toxic and hazardous to the environment when the concentration is beyond the acceptable limit11, 12, 13. Lead, Cadmium, Mercury and Arsenic have no advantageous effects in humans14. Low concentrations of heavy metals are known to have neurotoxic and carcinogenic actions15.
Permissible Limits of Heavy metals
World Health Organization (WHO) is answerable for people health and several public health problems, healthy diet, food security and nutrition. WHO and EPA are the top most agencies of health organization in the world. These agencies also provide a guideline for drinking water. WHO and EPA study the drinking water and provide the acceptable limit of various heavy metals and other parameters in drinking water. The table below shows the permissible limit of different toxic heavy metals.
Table 1: Permitted limit of heavy metals (WHO and EPA) in ppm 4, 13, 16
S.No. | Heavy metals | WHO (ppm) | USEPA (ppm) |
1. | Chromium | 0.05 | 0.05 |
2 | Iron | 0.3 | - |
3. | Cobalt | 0.10 | - |
4. | Nickel | 0.1 | - |
5. | Copper | 0.05 | 0.20 |
6. | Zinc | 5.0 | 5.0 |
7. | Arsenic | 0.05 | 0.01 |
8. | Cadmium | 0.005 | 0.005 |
9. | Mercury | 0.001 | 0.002 |
10. | Lead | 0.05 | 0.015 |
Applications of heavy metals
Chromium is used in the process of electroplating, leather tanning, metal finishing, textile industry and photography industry4. Chromium is used in nuclear power plants, data storage and also in metallurgical processes15. Iron is used for coating metal corrosion and steel industry mining16. Cobalt is used as a semiconductor. Co(II) is used in nuclear power plants, enamel painting on glass, manufacturing of aerospace materials, batteries and in the synthesis of vitamin B12 4.
Nickel is used in non-ferrous alloys, superalloy, electroplating industries, rechargeable batteries, catalysts, microphone capsules4, 6. Copper is used in mining operations, pharmaceutical equipment manufacturing, paper industry and kitchenware manufacturing17. Zinc is used in batteries, anti-corrosion coating, cans, paints, cosmetics, rubber industries, pigments and as Zn alloys18.
Arsenic is used in the industries of pesticides, mining, insecticides, ceramics, metallurgy, textile, veterinary medicine productions and also in the tanning process15. Cadmium is used in welding and electroplating processes. Cadmium plays a role in petroleum retaining, plastic stabilizers, coal combustion and nuclear fission plants4. Mercury is used in batteries, dental amalgams, photography, fuel combustion, pharmaceutical industries and textiles. It is also used as catalyst rectifiers, Hg vapour lamps and fungicides19. Lead is used in the production of electronic products, paints and also used in the electroplating process20. Lead also plays a role in the production of bullets and shot materials for soldiers 14.
Table 2: Applications of Heavy metals.
S No | Heavy metals | Industrial application | Biological application |
1. | Chromium | Electroplating, leather tanning, metal finishing, textile industry and photography industry 17, nuclear power plants, data storage, metallurgy processes 21. | Glucose metabolism17, lipid metabolism |
2. | Iron | Coating metal corrosion, steel industry, mining22, missionary, hospital equipments23 | Helps in photosynthesis, nutrient for phytoplankton26 Blood production, |
3. | Cobalt | Semiconductor, nuclear power plants, enamel painting on glass, aerospace materials, batteries25 | Formation of vitamin B12 4 Promote angiogenesis, erythropoiesis and anaerobic metabolism24 |
4. | Nickel | Non-ferrous alloys, super alloy, electroplating industries16 Rechargeable batteries, catalysts, microphone 21 | Capsules4 |
5. | Copper | Mining operations, pharmaceutical equipment, paper industry, kitchenware 7 | Cofactor for enzymatic reactions4, Radiotherapy of cancer, killing of cancer cells25 |
6. | Zinc | Batteries, anti-corrosion coating, cans, paints, cosmetics, rubber, industries, pigments, Zn alloys 15 | Absorption of calcium in the bones, creation of connective tissues, function of cell membrane |
7. | Arsenic | Pesticides, mining, insecticides, ceramics, metallurgy, textile, tanning process7 | Veterinary medicine productions15 |
8. | Cadmium | Welding, electroplating, petroleum retaining, plastic stabilizers, coal combustion, nuclear fission plants 20 | No known biological function |
9. | Mercury | Batteries, dental amalgams, photography, fuel combustion, pharmaceutical, textile, catalyst rectifiers, Hg vapour lamps, fungicides 22 | No known biological role |
10. | Lead | Electronic products, metal processing, paints, pigments, electroplating process4, production of bullets and shot materials for soldiers14 | No known biological role |
Toxicity and impacts
Various health-related pollutants, especially heavy metals surges in the environment due to their non-degradable, persistent and bioaccumulative nature. Long-term consumption and frequent exposure to toxic heavy metals can result in severe health problems 9. Heavy metals such as zinc subgroup ions, Hg2+ and Cd2+ will lead to serious extortions to human health because they are unsafe and have carcinogenic nature 19,20.
Chromium
Chromium (VI) in water are dangerous to the environment because of its high toxicity. Hexavalent- Chromium holds a high risk due to its water-soluble nature to enter into the living cells and cause acute health issues. It causes headache, diarrhoea, nausea, vomiting, carcinogenic and ulcer oedema24, skin rashes and irritation, weakness of immune systems and genetic constituents 9. It also produces lung tumours, allergic dermatitis 22. It can cause health disorders such as haemorrhage, pulmonary congestion and it affects human physiology 13.
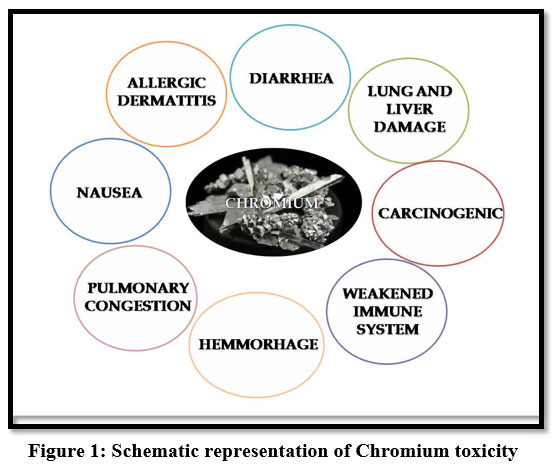 | Figure 1: Schematic representation of Chromium toxicity
|
Iron
Iron shows a major role in the biological system of complex formation in hemoglobin 27. High levels of iron cause side effects such as vomiting sensation, diarrhea or stomach upset 15.
Cobalt
Exposure to high amounts of cobalt may cause problems to fetal development during pregnancy and sometimes it is carcinogenic 18. Cobalt may lead to physical and mental problems like vomiting, asthma, kidney congestion, skin degeneration and weight loss 15.
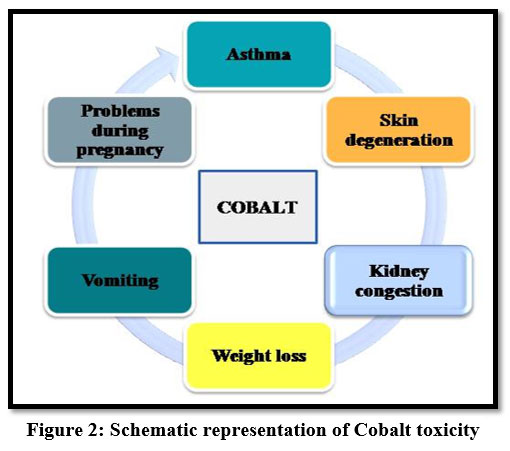 | Figure 2: Schematic representation of Cobalt toxicity
|
Nickel
Toxic effects of Nickel cause damage to internal organs and mental and neural systems. Ni2+ is fatal to sensitive species of water living organisms 21, 28. It causes dermatitis, headache, dizziness, nausea, vomiting.
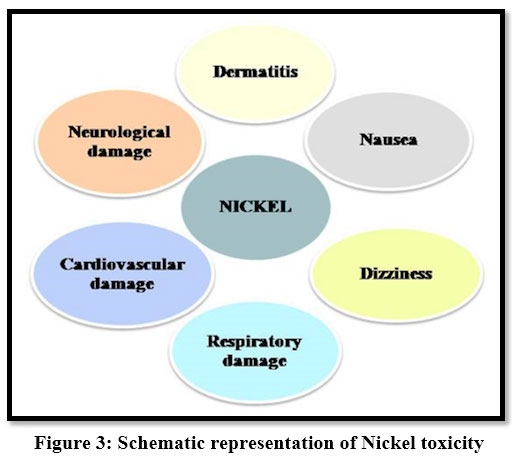 | Figure 3: Schematic representation of Nickel toxicity.
|
Copper
If the copper intake is in excess, it accumulates in the liver causing gastrointestinal problems, kidney damage and anaemia 11. The toxicity of copper leads to hair loss, headache 12, liver failure, Wilson's disease and Insomnia 9,11,14 . It also causes renal disorder and irritate the mouth, and also causes stomach ache, vomiting, diarrhea and headache 22.
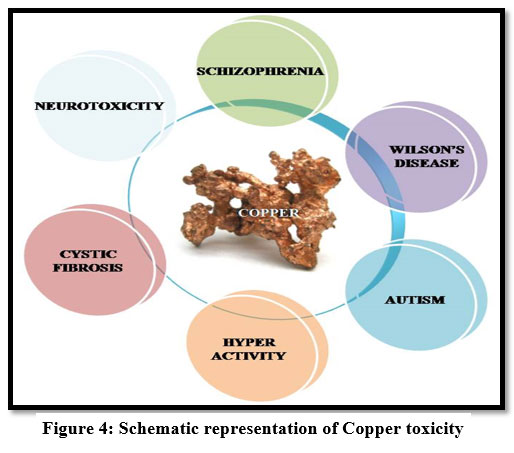 | Figure: 4 Schematic representation of Copper toxicity.
|
Zinc
Zinc is non-biodegradable and highly toxic and has a reverse effect on humans. High exposure to zinc causes depression, lethargy and increased thirst 7. And it also causes skin irritations, stomach cramps, anemia4 and bloody urine11. It leads to gastrointestinal distress and metal fume fever 9.
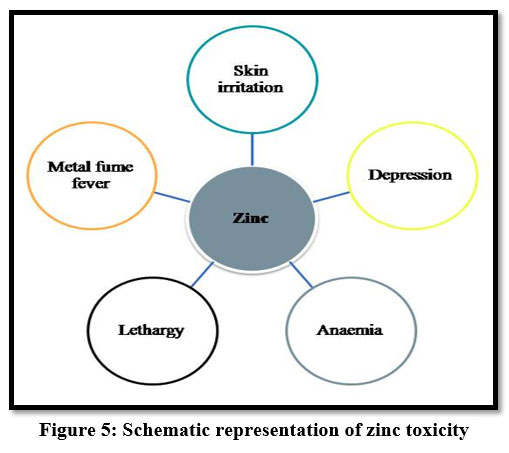 | Figure 5: Schematic representation of zinc toxicity.
|
Arsenic
Exposure to arsenic affects the internal organs of the human body. Long term exposure causes skin, lung, kidney and bladder cancer 14. And also it causes muscle weakness, neurological disorders 13.
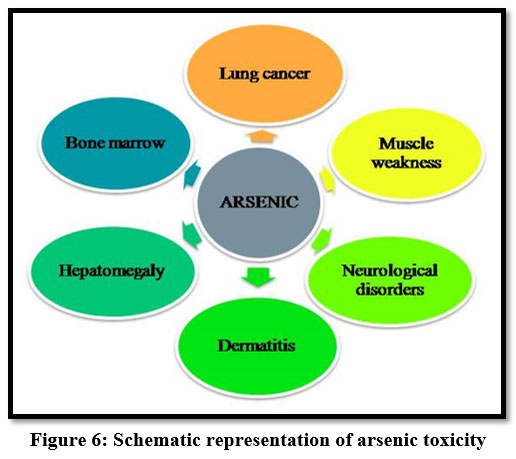 | Figure 6: Schematic representation of arsenic toxicity
|
Cadmium
Itai-Itai disease is caused because of chronic cadmium toxicity. Cadmium induces gastrointestinal disorder, bronchitis, hypertension23 and it is a human carcinogen, emphysema9, dyspnea and weight loss. Itai-Itai disease also causes cancer on lung, kidney liver and reproductive organs 13, 21, 27.
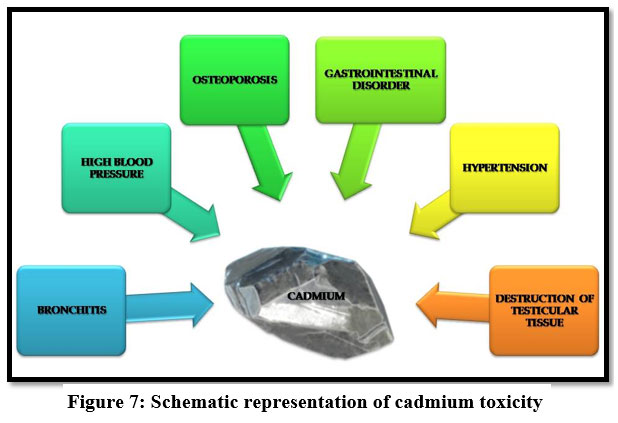 | Figure 7: Schematic representation of cadmium toxicity.
|
Mercury
Mercury causes retardation, genetic defects, teratogenic effects, rheumatoid arthritis, protoplasm poisoning 16, 17, 22. It is a neurotoxin and high concentration of mercury causes dyspnea15, abnormal irritation, acrodynia, gingivitis, stomatitis, neurological disorders and congenital malfunction 29. Inorganic mercury causes spontaneous abortion and hematochezia21.
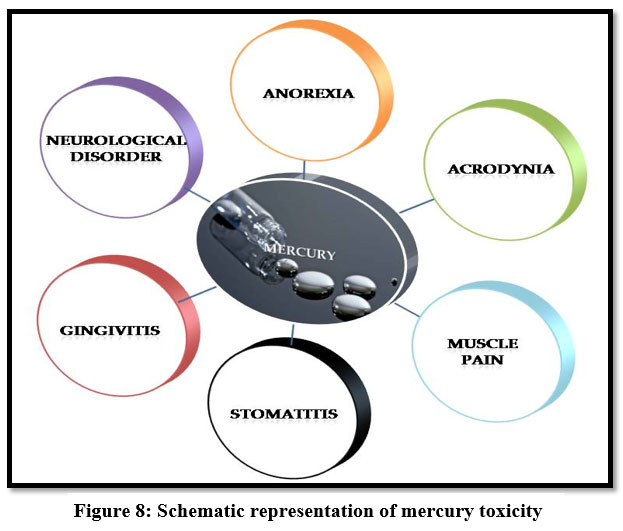 | Figure 8: Schematic representation of mercury toxicity.
|
Lead
Lead poisoning has been accepted as one of the major public health risks. Pb2+ accumulates mainly in bones, kidneys, brain and muscle, acute and chronic damage to the central nervous system and peripheral nervous system 28. It may cause anaemia, headache, irritability and muscle weakness, insomnia, hallucination, renal damage, dizziness, Malaise and anorexia30. Series effect of lead toxicity of its teratogenic effect, gastrointestinal damage. Children are sensitive to this metal and leads to high growth rate and metabolism and mental retardation 13.
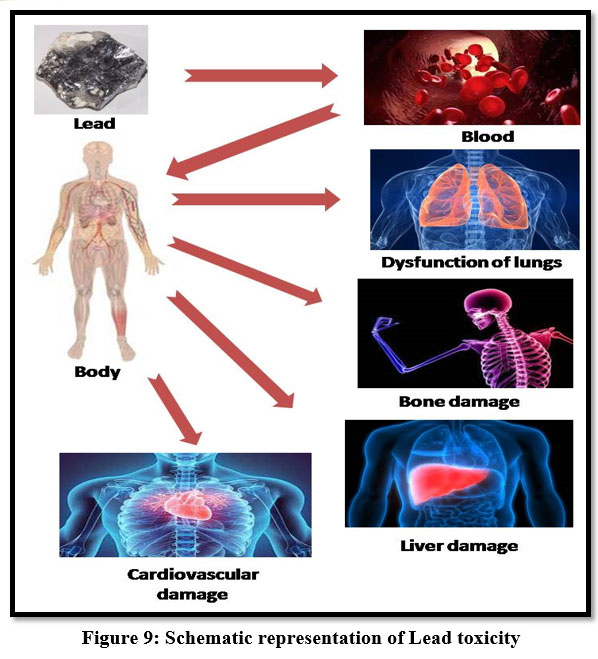 | Figure 9: Schematic representation of Lead toxicity
|
Sources of heavy metals in water samples
Heavy metal pollution continuously increases in the environment due to industrial development and urbanization. Due to the rapid development of industries, mining, electroplating, batteries, and paper industries, huge amounts of toxic metals are getting discharged into the environment. The metal plating industry is one of the major source that shed large amounts of wastewaters. Metal surface treatment processes and electroplating generate large quantities of wastewater containing heavy metals.
Chromium
The main source by which chromium entered into the environment is several industrial actions such as metal smelting, tanning, electroplating, metallurgy, steel industries 23. It is also released from dyes, textile industry and leather industry 20,21.
Iron
The major source of iron are natural deposits, wastes from metal processing industries, ores, and corrosion of vessels and rods 24.
Cobalt
Cobalt is released from nuclear power plants and industries like petrochemical, metallurgical, electronics, dyes 14.
Nickel
Nickel is spread via industrial process batteries manufacturing paint, mining, metal finishing & forging 17. Petroleum refining, electroplating are also other sources of nickel contamination 13.
Copper
Main sources of copper in industrial discharge are paints and pigments fertilizer industries, pulp, wood pulp production, metal cleaning, paperboard mills, mining, batteries, copper cooking pots 20, 27 . As a pollutant in food specifically shellfish, mushroom, chocolate, liver, nuts, electroplating industry 22.
Zinc
Sources of zinc include steel processing, mining and coal combustion, brass metal works, paper and pulp industries 30.
Arsenic
The major source of Arsenic in water is via dissolution of minerals, ores, sediments, bio- organisms, rocks, ground water, combustion of fossil fuels, mining and pesticides 19.
Cadmium
Cadmium is released via nuclear fission plants, refining pesticides, mining, plants and plastics, Cd and Ni batteries, fertilizers and electroplating 29. It is also emitted by metal smelters and tobacco smoke 13.
Mercury
Liberation of mercury into environments through the mining paper & paint industries 30. And also includes fertilizer industries, Batteries, Textile photographic fossil fuel combustion, waste incineration scientific instruments, pharmaceutical industries 4, 22. Agriculture industries, pulp and paper preservatives, catalysts in organic synthesis are also other means of mercury liberation into the environment 13.
Lead
Lead enters into the atmosphere from windblown dust, biogenic material 28. Other sources of lead are industrial old lead pigment paints, fuel, mining sources, leaded gasoline, batteries, explosives, electroplating, glass manufacturing industries 16, 19, 22.
Table 3: Sources of Heavy metals 20, 22, 28
S.No | Sources | Heavy Metals |
1. | Mining | Cr, Ni, Cu, Zn, As, Cd, Hg, Pb |
2. | Electroplating | Cr, Ni, Cd, Pb |
3. | Textile Industry | Cr, Hg |
4. | Industrial waste | Fe, Cu |
5. | Petrochemical Industries | Ni, Co |
6. | Batteries | Ni, Cu, Hg, Pb, Cd |
Methodology used for the elimination of heavy metals
Removal of toxic heavy metals from wastewater has become a major concern nowadays. Unproductive ways of dealing heavy metal ions may cause long-term risk to the ecosystem and humans. Number of techniques for the efficient elimination of heavy metals from wastewater are available.
Chemical precipitation
This is the physicochemical process and a very flexible approach to various pollutants removals. In chemical precipitation, a reagent is added which reacted with heavy metal ions and resulting in the formation of insoluble compounds or insoluble precipitates 29. Additional methods like sedimentation and filtration are required to remove the precipitates 29. To reduce the solubility of the dissolved pollutants, it can be carried out by lowering the temperature or by adding some chemicals like sodium bicarbonate and ferric chloride to the solution23. But chemical precipitation is not preferred due to increased cost. Chemical precipitation is applied for chromium and nickel plating industries 24. The removal of the chemical precipitation process depends on pH, charge of the ions, temperature18, alkalinity, concentration of metal ions and phosphate level 33.
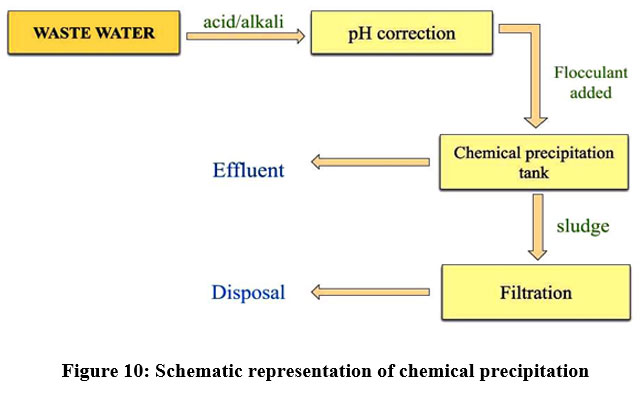 | Figure 10: Schematic representation of chemical precipitation
|
Hydroxide precipitation
Heavy metal ions have low solubility in alkaline medium, thus the precipitation technique is often organised in alkaline condition 26. Lime, Limestone 29 and NaOH 33 are frequently used precipitants because they are cheap and available easily. Sodium carbonate, Calcium hydroxide , Ammonium hydroxide 33, Magnesium hydroxide and Calcium chloride are other available precipitants used for the formation of hydroxide precipitates.
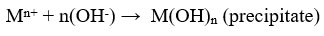
where Mn+ denotes the metal ions, OH- ions denotes the precipitants and M(OH)n denotes the insoluble metal hydroxide precipitate 33. Cr is removed using NaOH as precipitants 26. Removal efficiency of Zinc is 99.3% by using lime as a precipitant 23. The commonly used method is hydroxide treatment because cost of precipitant is cheap, relative simplicity and ease of automatic pH control34. Some metal hydroxides are amphoteric, that leads to secondary precipitation in solution33.
Sulphide precipitation
This method is frequently used method and precipitants are mostly FeS, CaS (solid)34, BaS, Na2S2O328, aqueous precipitants like NaHS, (NH4)2S and Na2S 23. Sulphide precipitates are non- amphoteric, so it has high removal efficiency 29.
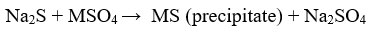
The sulphide precipitation overcomes the hydroxide precipitation because the solubility of sulphide precipitates are lower than the hydroxide precipitates33. Other advantages are rapid reaction rate, better setting properties33, reuse of sulphide precipitates by smelting and selective removal of metal ions23. The factors that influence the sulphide precipitation are pH, type of sulphur source, initial metal concentration, the precipitation agent, ratio of the two components 32.
Formation of colloidal precipitates 29, toxic fumes of Hydrogen sulphide 33 are the disadvantages of sulphide precipitation. It is not a conventional method for the mine water treatment, but it is widely used to eliminate heavy metals from the metal finishing industry. This process removes heavy metals like Lead, Chromium, Zinc, Nickel, cadmium and Mercury 36.
Adsorption
A surface phenomenon where atoms or ions occupy to the active site of adsorbent, creating a layer of the adsorbate over the surface of the adsorbent is known as adsorption18. Adsorption is an effective purification and separation technique used in industries due to its flexibility in design and ease of operation 37. Adsorption is a physicochemical technique extensively applied to eliminate heavy metals from the wastewater through the interaction between the adsorbents and pollutants 39. Adsorption process increases with proliferation in the surface area of adsorbents 40. The interaction between the adsorbent and adsorbate are classified into physical and chemical adsorption 39. A very weak Vander Waals force of attraction holds the adsorbate particles and adsorbent and the process is called physisorption and it has low adsorption capacity41,42. If the adsorbate and adsorbent are bound due to chemical bonds like covalent or ionic, then it is chemisorption43. Chemisorption has high efficiency but causes secondary pollution 45, 46, 47.
Generally, when pollutants get adsorbed on the solid adsorbents, there are three steps involved 49,52:
The transference of the adsorbate from the bulk solution to the surface of the adsorbent
Adsorption on the surface of the adsorbent
Transport of pollutants within the adsorbent
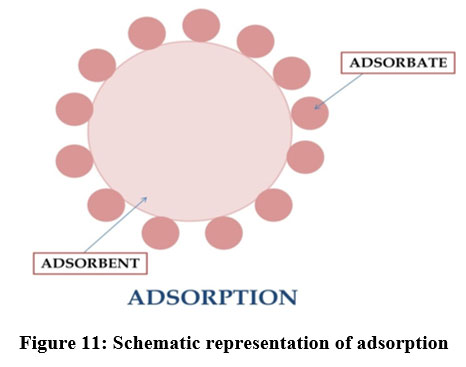 | Figure 11: Schematic representation of adsorption.
|
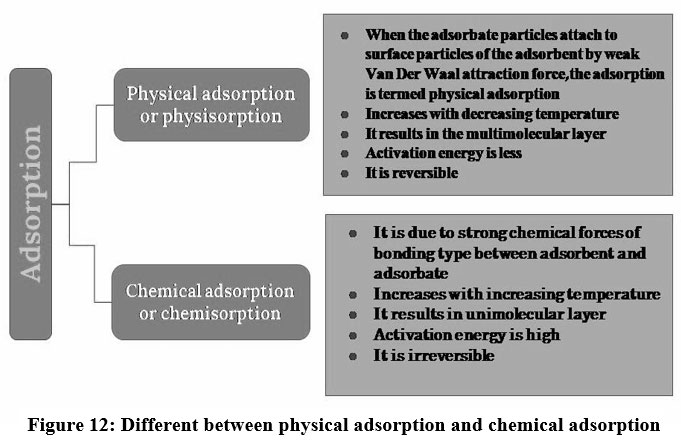 | Figure 12: Different between physical adsorption and chemical adsorption.
|
All substances have an adsorption effect. But selection of adsorbents for the effective removal is an important component 50. Adsorbents should have strong adsorption capacity, low equilibrium solubility, good selectivity and stability, ease of desorption52. The adsorption depends on time, quantity and nature of adsorbent, temperature, concentration of adsorbate, pH, etc.54.
Depending on the amount of adsorbate desorbed from the adsorbent surface, the reversibility of the adsorption process can be measured 55, 56.
Adsorbents
To make an effective adsorption process, the selected adsorbent should be readily available, inexpensive, eco-friendly and abundant in nature58. The elimination of heavy metals utilizing cheaper adsorbents is developing in recent days. There are several cheap adsorbents resulting from manufacturing byproducts, farm waste, biomaterials, reformed biopolymers, etc. that are slightly modified to effectively remove heavy metals from polluted wastewater52. Modified natural materials are used for adsorption such as clay, peat moss, zeolite, etc. Clinoptilolite and Peat moss are the most frequently used zeolite due to its specificity to some heavy metals such as lead, copper, cadmium and zinc 34. Calcinated phosphates are used to adsorb Pb2+, Cu2+ and Zn2+7.
Adsorption using biological wastes and modified agricultural wastes is called Biosorption. Orange peel is effectively used for the removal Ni2+7. Cr6+ are removed using almond shell, neem leaf powder9, crushed coconut shell, hazelnut shell, palm flower 17, groundnut hull 29, activated rice husk 56, UlvaLactuca 29, rose wood 37, eggshells 37, etc. Cu2+ are adsorbed using sesame husk81, tea waste59, marine green macroalgae49, UlvaLactuca49, etc. Musa paradisiaca peels are effevtively utilised to remove of Cd2+ and Pb2+ 54. Some biosorbents utilized to remove heavy metals effectively are, CoriandrumSativum, grape stalk wastes34, coffee husk, sugar beet peel in gels, citrus peels, potato peels 49,56,59,60. Agricultural waste is the most preferred adsorbent due to several reasons such as availability in abundance, economic and ecofriendly, efficient, unique chemical composition and low cost 54. Heavy metals are also removed by using industrial solid wastes 58,61. Cement bypass is used to treat tanneries effluent wastewater 60. Treated waste newspaper has 72% efficiency to remove Chromium(VI)38. Zn2+are removed using powdered waste sludge, dried marine green macroalgae61.
Ion exchange process
The phenomenon used in the ion exchange process is, it draws dissolved ions present in the liquid state to the solid state62. This method is utilized only for low concentration of metals present in the solution and it is extremely sensitive to the pH of the aqueous phase. The resin eliminates metal ions in the dissolved form and releases other ions of like charges without altering the internal structure of the resin 62,63. In the cationic resins H+ and Na+ are exchanged with Ni, Cu and Zn ions in the same resin. Similarly hydroxyl and chloride ions present in the anionic resin can be replaced by the chromate, sulphate, NO3-, CN- and DOC present in the solution64. Cation exchange resins called IRN77 and SKNI removes 95% of Chromium(III) from wastewater18. Among all the available kinds, synthetic polymers were preferred such as styrene- divinylbenzene, gel-like resins, macropore resin, etc.26.
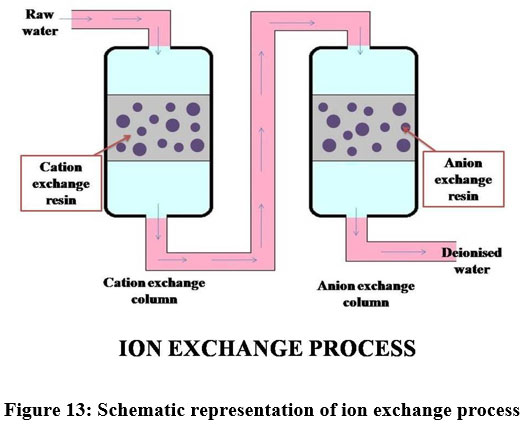 | Figure 13: Schematic representation of ion exchange process.
|
Membrane process
The membrane filtration process has been increasingly used to treat wastewater due to its convenient operation69. A membrane adsorbent is ready by joining the functional groups to the surface and pore walls of the membrane. The aimed pollutants are preferentially adsorbed to the functional group site. When the polluted water moves via the membrane the functional active binding sites will be linked with the target pollutant to remove contaminants with a high adsorption rate and capacity71. Generally three types of membrane with minor differences are. There are many parameters that can disturb the membrane process like used materials, pore size and composition, which displays highly competent and feasible separation of heavy metals 73. Membrane filtration is the most economic process because the water obtained from this process is of ultrapure water. Membrane process was also coupled with other techniques like ion- exchange process, adsorption, etc., 70. To achieve the required filtration, membranes like cellulose, zeolite, polyamide, polyester, natural mineral based ceramic membranes and carbon membranes are used 69.
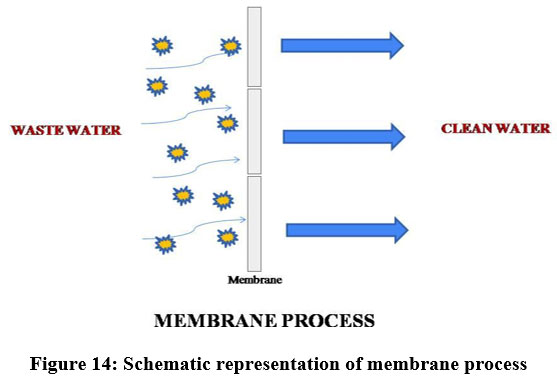 | Figure 14: Schematic representation of membrane process.
|
There are several membranes available to remove heavy metals.
Cd is removed by montmorillonite kaolin, tobermorite, magnetitesiligagili and alumina membranes
Cu is removed by chitosan nanoparticles embedded ketoglutaric acid membranes
Lead is removed by polymeric cation exchanger having nano- Zr
Arsenic removed by acid modified carbon membranes 61
Ultrafiltration
Ultrafiltration uses permeable membranes to distinct heavy metals and suspended solids (1000-10000 Da) on the basis of its pore size stretching from 5 to 20 nm. Ultrafiltration 30 is mainly used for product recovery and pollution control in the food, chemical, electronics, pharmaceuticals, metal plating industries due to its lower driving force, lesser space necessity and high loading density 29. More than 90% of removal efficiency can achieve by UF 70.
Reverse osmosis
A process that utilizes pressure to move the solution through a membrane that has solute on one side and permits the pure solvent on the other side is known as Reverse osmosis (RO). RO is dependent on the solute concentration, pH, pressure and water flux rate32. According to various studies from the literature, the removal percentage of reverse osmosis has achieved 99.9% 72.
Nanofiltration
Nanofiltration performs separation between RO and UF. The molecular weight of the solute ranging from 200 to 1000 Da is rejected by a nanofiltration membrane with pore size ranging from 0.5 to 2 nm74. Analysing the comparative study of RO and NF, RO performs superior than NF in wastewater separation due to its anti- compressing ability of membrane75. Copper, Arsenic, Nickel and Chromium are removed from this process 37.
Solvent Extraction
The relocation of material from one solvent to another solvent in which the distribution coefficient is different is referred by extraction. Majority of the material can be extracted by repeated extraction22. Solvent Extraction is the cost effective energy efficient process to extract heavy metals from various complex leach products and industrial effluents using organic solvents 29. Organic solvents like acetone, ethanol, hexane, methanol, acetonitrile, etc., are the most commonly used solvents 24. Solvent extraction is widely used in the field of biomedical industries, inorganic, organic chemistry labs and wastewater treatment 10. The solvent extraction process is used to separate metals like Zinc 78, Nickel, lead, chromium 30 and Cobalt 34.
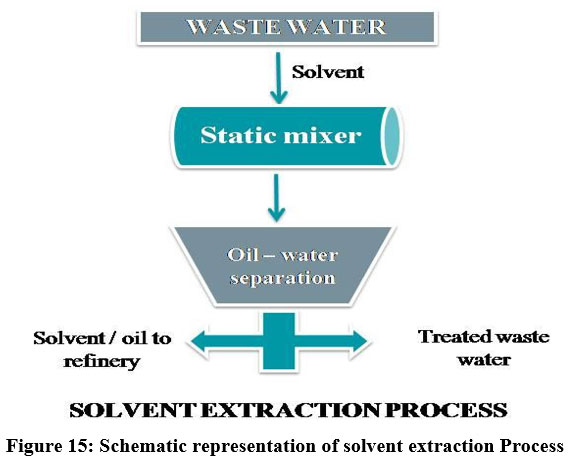 | Figure 15: Schematic representation of solvent extraction Process.
|
Electrocoagulation
Electrocoagulation (EC) is an electrochemical approach that uses electrical current to remove metals from the solution. The oxidation occurs at anode and reduction occurs at cathode when electricity is applied 81. Electrocoagulation is one of the most promising techniques in the present era due to its high removal efficiency81. This method is utilized to remove heavy metals from industries like steam cleaners, municipal sewage, palm oil effluents, pressure washers 82, electroplating, laundry, restaurant, poultry slaughterhouse 82, textile, paper, acid mine drainage and organic matters 82. Electrocoagulation process is used to remove suspended solids, dissolved metals, tanninsand dyes. EC process is able to eliminate heavy metals like Cr, Cd, Zn, Ni, Hg and Co 83. pH, current density, conductivity 83, gap between the electrodes 88 and treatment time are the major factors that affect the performance of EC process 89. Aluminium and Iron plates are widely used as sacrificial electrodes in EC processes 61,66. But the usage of less reactive metal alloys like magnesium and aluminium alloy is a new trend 84. Arsenic and Chromium are effectively removed using Iron electrode rather than Aluminium electrode 85. By adjusting the pH and operating conditions, Cr (75%), Cu (97%), Zn (100%), and Ni (90%) removal efficiencies were obtained 85.
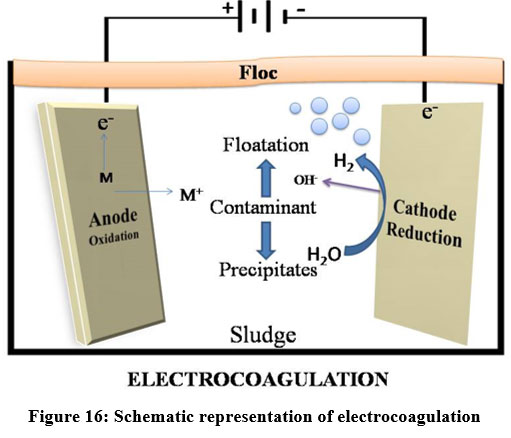 | Figure 16: Schematic representation of electrocoagulation.
|
Electrocoagulation mechanism
Two electrodes were taken and current was applied between them and thereby the dissolution of the sacrificial anodes occurs and the supply ions to the wastewater was done by electrocoagulation. It allows settled, coagulated or soluble contaminants to develop aggregates86. This process depends on the nature of aqueous solution, specifically conductivity 86.
Steps involved in Electrocoagulation process are:
Oxidation of the “sacrificial electrodes”
Water molecules reduced at the negative electrode
The formed ions migrate to the oppositely charged electrodes
Metallic hydroxides are formed by the interaction of the positive ion and hydroxyl ion
Larger aggregates are formed by the absorption of pollutants into the hydroxide structures
Removal of aggregates by flotation 7
Table 4: Heavy metal removal methodologies –uses and limitations
S. No | Techniques | Advantages | Disadvantages |
1 | Adsorption | Highly effective, low cost4, flexibility in design27, ease of operation10, eco- friendly | High production of waste products, Low selectivity4 |
2 | Ion exchange process | Low energy requirements 26, reversible, less sludge production 18, selectivity 10 | High cost process, resin does not exist for all heavy metals, not applicable for a large scale system, produce secondary pollutant 18 |
3 | Membrane process | Small space requirement, low pressure 18, high separation selectivity7 | Expensive7, complex process 23 |
4 | Solvent extraction | Speed, simplicity21, less implementation time10 | High cost and selective 10 |
5 | Electro-coagulation | High efficiency, easy to handle17, eco-friendly25 | Large source requirement of electricity17, high investment 25 |
Reuse of treated water
Water is an invaluable commodity that was once available almost for free cost. Water fulfills several needs in almost every industry. The utilised water from the industry comes out as wastewater with hazardous pollutants and leads the environment to danger. Wastewater is treated with various effective techniques 94. The treated wastewater is almost free from pollutants and heavy metals are removed to the maximum extent. Reuse of the treated wastewater depends on factors such as waste volume, percentage of raw water, effluent standards, operation and maintenance costs. In the present era, reuse of treated wastewater is becoming a trend 96. The applications of purified wastewater includes washing, coating, cooling, spraying, boiler water make-up etc. Treated wastewater by Electrocoagulation is used for recoveries of Pb and Zn 92. Treated textile wastewater is used for the irrigation of container grown ornamental shrubs 97. Treated effluents from the dairy industry are used in cooling towers or boilers, washing the floors and external parts of trucks and rinsing outside areas in the dairy industry 96.
Summary
This review article elaborates on the properties and role of heavy metals in various fields. On the basis of the study, Mercury and Chromium (VI) is the most toxic heavy metal that contaminates the environment from various sources. Iron does not cause much impact on the environment and human beings. Mining is the major source that releases many heavy metals into the environment. Various industries like textile, battery, electroplating are the other major sources that cause heavy metal pollution. The heavy metal contaminated wastewater is treated using various techniques like ion-exchange, adsorption, solvent extraction, electrocoagulation, membrane filtration and chemical precipitation. Among these techniques, Adsorption technique is used widely because of its simplicity. From the recent studies on adsorption, bio-sorbents are the extensively used adsorbent because of its easy availability. Among the three membrane filtration processes, reverse osmosis has a high removal efficiency of 99.9%. Electrocoagulation process has 90% removal efficiency to remove copper. 90% of Cr is removed using the ion- exchange process. Reuse of treated wastewater has various applications like growing shrubs, washing and cleaning.
Acknowledgement
The authors greatly acknowledge the Principal, Management and Department of Chemistry of Lady Doak College, Madurai for the support rendered for the completion of the work.
Funding sources
The author(s) received no financial support for the research, authorship, and/or publication of this review article.
Conflict of Interest
The authors declare no conflict of interest.
Authors’ Contribution
All the authors involved in reviewing and preparing the content for the review article.
Data Availability Statement
All data underlying the results are available as part of the article and no additional source data are required.
Ethics Approval Statement
Ethical review and approval were not required for the present review.
Reference
- Raafat Abdeldayem, A preliminary study of heavy metals pollution risk in water, Applied Water Science, 2020; 10(1): 1-4.
CrossRef - Olubunmi G. Abatan, Peter A. Alaba, Babalola A. Oni, Akpojevwe K, Efeovbokhan V., Abnisa F., Performance of eggshells powder as an adsorbent for adsorption of hexavalent chromium and cadmium from wastewater, SN Applied Sciences, 2020; 2: 1996-2001.
CrossRef - Abdelfattah I., Adel A. Ismail, Al Sayed F., Almedolab A., Aboelghait K.M., Biosorption of heavy metals ions in real industrial wastewater using peanut husk as efficient and cost effective adsorbent, Environmental Nanotechnology, Monitoring & Management, 2016 ; 6: 176-183.
CrossRef - Mahmud H.N.M.E., ObidulHuq A.K., RosiyahbintiYahya, The removal of heavy metal ions from wastewater/aqueous solution using polypyrrole-based adsorbents: A review, The Royal Society of Chemistry, 2016; 6: 14778-14791.
CrossRef - Khoso W.A., Haleem N., AnwerBaig M. , Yousuf Jamal, Synthesis, Characterization and heavy metal removal efficiency of nickel ferrite nanoparticles, Scientific Reports, 2021;11: 3790-3800.
CrossRef - Zhang W., Yuhong An, Shujing Li, Zhechen Liu, Zhangjing Chen, YukunRen, Wang S., Chang X., Ximing Wang, Enhanced heavy metal removal from an aqueous environment using an eco-friendly and sustainable adsorbent, Scientific Reports, 2020;10:16453-16472.
CrossRef - Ming Hua, Zhang S., Bingcai Pan, Zhang W., Lu Lv, Zhang Q., Heavy metal removal from water/wastewater by nanosized metal oxides: A review, Journal of Hazardous Materials 2012; 211(212): 317-331.
CrossRef - Hegazi H.A., Removal of heavy metals from wastewater using agricultural and industrial wastes as adsorbents, HBRC journal, 2013; 9: 276-282.
CrossRef - Sinh Vo T., Hossain M.M., Jeong H.M., Kim K., Heavy Metal removal applications using adsorptive membranes, Nano Convergence, 2020; 7: 36-62.
CrossRef - Nagger N.A., Ragaa A. Hamouda, Amna A. Saddiq , Monage H. Alkinani, Simultaneous bioremediation of cationic copper ions and anionic methyl orange azo dye by brown marine alga fucusvesiculosus, Scientific Reports, 2021; 11: 3555-3574.
CrossRef - Liu J, Chen J, Huang L, Heavy metal removal from MSS fly ash by thermal and chlorination treatments, Scientific Reports, 2015; 5:17270-17285.
CrossRef - Fenglian Fu, Wang Q, Removal of heavy metal ions from wastewaters: A review, Journal of Environmental Management, 2011; 92: 407-418.
CrossRef - Wo?owiec M., Kaufman M.K., Pruss A., Rzepa G., Bajda T., Removal of heavy metals and metalloids from water using drinking water treatment residuals as adsorbents: A Review, Minerals, 2019; 9(8): 487-504.
CrossRef - Neisan, R.S., Saady, N.M.C., Bazan, C., Zendehboudi, S., Al-nayili, A., Abbassi, B., Chatterjee, P.Arsenic Removal by Adsorbents from Water for Small Communities’ Decentralized Systems: Performance, Characterization, and Effective Parameters Clean Technologies, 2023; 5 (1): 352-402.
CrossRef - Sreelakshmi C.D, Heavy metal removal from wastewater using Ocimum Sanctum, International Journal of Latest Technology in Engineering, Management & Applied Science, 2017; 6(4): 85-90.
- Sureshkumar Halnor, Removal of heavy metals from wastewater: A review, International Journal of application or innovation in Engineering & Management, 2015; 4(10): 9-22.
- Al-Qadah Z., Al-Shannag M., Heavy metal ions removal from wastewater using electrocoagulation process: A comprehensive review, Separation Science And Technology, 2017; 52(17): 2649 - 2676.
CrossRef - Soliman N.K., Moustafa A.F., Industrial solid waste for heavy metals adsorption features and challenges; A review , J Mater Res Technol., 2020, 9(5): 10235-10253.
CrossRef - Mudhoo A, Vinod K. Garg , Wang S., Removal of heavy metals by biosorption, Environ Chem Lett., 2012; 10: 109-117.
CrossRef - Celebi H., Gok G., Gok O., Adsorption capability of brewed tea waste in waters containing toxiclead (II), cadmium(II), nickel(II), and zinc (II)heavy metal ions, Scientific Reports, 2020; 10: 17570-17592.
CrossRef - Vani Sharma, Padma Singh, Heavy metals pollution and its effect on environment and human health, International journal of recent scientific research, 2015 ; 6(12): 7752-7755.
- Tripathi A., RawatRanjan M., Heavy metal removal from wastewater using low cost adsorbents, J bioremed. Biodeg. 2015; 6(6): 315-320.
CrossRef - Podesva P., Gablech I., Neuzil P., Nanostructured gold microelectrode array for ultrasensitive detection of heavy metal contamination, Analytical chemistry, 2018; 90(2): 1161-1167.
CrossRef - Barakat M.A., New trends in removing heavy metals from industrial wastewater, Arabian journal of chemistry, 2011; 4: 361-377.
CrossRef - Bingbing Li, Zhou F., Huang k., Wang Y., Mei S., Zhou Y., Jing T., Environmentally friendly chitosan/PEI- grafted magnetic gelatin for the highly effective removal of heavy metals from drinking water, Scientific reports, 2017; 7: 43082-43090.
CrossRef - Peng H., Guo J., Removal of chromium from wastewater by membrane filtration, chemical precipitation, ion exchange, adsorption, electrocoagulation, electrochemical reduction, electrodialysis, nanotechnology: a Review, Environmental chemistry letters, 2020; 18: 2055-2068.
CrossRef - Sun D.T., Li Peng, Washington S., Reeder, Moosavi S.M., Tianna D., Britt K.D., Oveisi E., Queen W.L., Rapid, Selective heavy metal removal from water by a Metal-Organic framework/Polydopamine Composite, ACS Cent.Sci., 2018; 4: 349-356.
CrossRef - Tunsu C., Wickman B., Effective removal of mercury from aqueous streams via electrochemical alloy formation on platinum, Nature Communications, 2018; 9: 4876-4885.
CrossRef - Carolin C. F., Kumar P. S., A. Saravanan, Joshiba G. J., Mu.Naushad, Efficient techniques for the removal of toxic heavy metals from aquatic environments: A review, Journal of Environmental Chemical engineering, 2017; 5: 2783-2799.
CrossRef - Ince M., Ince O. K., Heavy metal removal techniques using response surface methodology: Water / wastewater treatment, Biochemical toxicology -heavy metals and nanomaterials, 2019; 6: 1-19.
CrossRef - Jiayu Li, Zheng B, Yangzhuo He, Zhou Y., Che X., Ruan S., Yang Y., Dai C., Tang L., Antimony contamination, consequences and removal techniques: A review, Ecotoxicology and environmental Safety, 2018; 156: 125-134.
CrossRef - Gunatilake S.K., Method of removing heavy metals from industrial wastewater, Journal of Multidisciplinary Engineering Science Studies, 2015; 1(1): 12-18.
- Pohl A., Removal of heavy metal ions from water and wastewaters by sulfur-containing precipitation agents, Water air soil Pollut., 2020; 231: 503-520.
CrossRef - Samson O., Owalude, AdedibuC.Tella, Removal of hexavalent chromium from aqueous solutions by adsorption on modified groundnut hull, Beni-Suef University Journal of Basic and applied Sciences, 2016; 5: 377-388.
CrossRef - Xia M., Chen Z., Yao Li, Chuanhua Li, Ahmad N.M., Waqas A. Cheema , Zhu S., Removal of Hg(II)in aqueous solutions through physical and chemical adsorption principle , RSC Adv, 2019; 9 : 20941-20953.
CrossRef - Burakov A.E., Galunin E.V., Burakova I.V., Kucherova A.E., Agarwal S., Tkachev A.G., Gupta V.K., Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes;A review, Ecotoxicology and Environmental Safety, 2018; 148: 702-712.
CrossRef - Malik D.S., Jain C.K., Yadav K.A., Removal of heavy metals from emerging cellulosic low-cost adsorbents: a review, Applied Water Science, 2017; 7: 2113-2136.
CrossRef - Kovacova Z., Demcak S., Balintova M., Removal of copper from water solutions by adsorption on spruce sawdust, Proceedings, 2019; 16(1): 52-56.
CrossRef - Dehghani M.H., Sanaei D., Ali I., Bhatnagar A., Removal of chromium (VI) from aqueous solution using treated waste newspaper as a low-cost adsorbent: Kinetic modeling and isotherm studies , Journal of Molecular Liquids, 2016; 215: 671-679.
CrossRef - Desai B., Desi H., Potential of moringaoleifera(drum sticks)seeds and its application as a natural adsorbent in removal of heavy metal ions , International Journal of Environment, Ecology, 2013; 3(4): 9-22.
CrossRef - Mishra N., Dr. Mishra N.K., Removal of toxic metal Cr(VI) from aqueous solutions using low cost adsorbent , Imperial Journal of Interdisciplinary Research, 2016; 2(10): 332-333.
- Khai M. Nguyen, Bien Q. Nguyen, Hai T. Nguyen , Ha T.H. Nguyen ,Adsorption of arsenic and heavy metals from solutions by unmodified iron-ore sludge, Applied Sciences, 2019; 9: 619-632.
CrossRef - Ibigbami T.B., Dawodu F.A., Akinyeye O.J., Removal of heavy metals from pharmaceutical industrial wastewater effluent by combination of adsorption and chemical Precipitation methods, American Journal of Applied Chemistry, 2016; 4(1): 24-32.
CrossRef - Hussein S. Mohamed , Soliman N.K., Doaa A. Abdelrheem, Arwa A. Ramadan, Ahmed H. Elghandour, Sayed A. Ahmed, Adsorption of Cd2+ and Cr3+ ions from aqueous solutions by using residue of Padina gymnospora waste as promising low cost adsorbent, Heliyon, 2019; 5: 01287-01319.
CrossRef - Ouyang D., Zhuo Y., Liang Hu, Zeng Q., Yuehua Hu And Zhiguo He, Research on the adsorption behaviours of heavy metal ions by porous material prepared with silicate tailings, Minerals, 2019; 9: 291-307.
CrossRef - Seo-Yun Lee, Hee-Jeong Choi, Persimmon leaf bio-waste for adsorptive removal of heavy metals from aqueous solution, Journal of Environmental Management, 2018; 209: 382-392.
CrossRef - Lim A.P., Aris A.Z., A review on economically adsorbents on heavy metals removal in water and wastewater, Rev Environ Sci Biotechnol., 2014;13: 163-181 .
CrossRef - Babel S., Kurniawan T.A, Cr(VI)removal from synthetic wastewater using coconut shell charcoal and commercial activated carbon modified with oxidizing agents and /or chitosan, Chemosphere, 2014; 54: 951-967.
CrossRef - Manjuladevi M., Anitha R., Manonmani S., Kinetic study on adsorption of Cr(VI), Ni(II),Cd(II) and Pb(II) ions from aqueous solutions using activated carbon prepared from cucumis melon peel , Applied Water Sciences, 2018; 8: 36-44.
CrossRef - Singh S., Tripathi A., Srivastava S.K., Removal of hexavalent Chromium from contaminated waters by mangiferaindica Seed powder (biosorption), IJSRD - International Journal for Scientific Research & Development, 2014; 1(12): 2559-2565.
- Ahmed S., Aktar S., Zaman S., A.Jahan, Md. Latiful Bari, Use of natural bio - sorbent in removing dye, heavy metal and antibiotic - resistant bacteria from industrial waste water, Applied Water Science, 2020; 10: 107-117.
CrossRef - Jain C.K., Malik D.S., Yadav A.K., Applicability of plant based biosorbents in the removal of heavy metals : A review , Environ. process, 2016; 3: 495-523.
CrossRef - Oladipupo O. Ogunleye, Mary A. Ajala, Samuel E. Agarry, Evaluation of biosorption capacity of banana (Musa paradisiaca stalk for lead (II) removal from aqueous solution, Journal of Environmental Protection, 2014; 5: 1451-1465.
CrossRef - El-Araby H.A., Abel M.M Ahmed Ibrahim, Mangood A. H. , Adel A.H. Abdel-Rahman, Sesame husk as adsorbent for copper(II) Ions removal from aqueous solution, Journal of Geoscience and Environment Protection, 2017; 5: 109-152.
CrossRef - IIbis N. E., Asoluka C.A., Use of agro - waste (musaparadisiaca peels) as a sustainable biosorbent for toxic metal ions removal from contaminated water, Chemistry International 2018; 4 (1): 52-59.
- Shan T.C, M. AI Matar, Essam A. Makky, Eman N. Ali, The use of MoringaOleifera seed as a natural coagulant for wastewater treatment and heavy metals removal, Appl. Water Sci., 2017; 7: 1369 -1376.
CrossRef - Chun-Xiang Cai, JianXu, Nian- Fang Deng, Xue-Wei Dong, Hao Tang, Yu Liang, Xian-Wei Fan, You-Zhi Li, A novel approach of utilization of the fungal conidia biomass to remove heavy meals from the aqueous solution through immobilization, Scientific Reports, 2016; 6: 36546-36558.
CrossRef - Peng H., Guo, Bingwing, Adsorption behaviour of Fe(III) on aqueous solution on melamine, Water Science & Technology, 2020; 82(9): 1848-1857.
CrossRef - Johnson E. Efome, Rana D., Matsuura T., Lan, Insight studies on metal-organic framework nanofibrous membrane adsorption and Activation for heavy metal ions removal from aqueous solution, ACS Appl. Mater. Interfaces, 2018; 10: 18619-18629.
CrossRef - Khulbe K.C., Matsuura T., Removal of heavy metals and pollutants by membrane adsorption techniques, Applied Water Science, 2018; 8: 19-49.
CrossRef - Huang X., Guida S., Jefferson B., Ana Soares, Economic evaluation of ion - exchange processes for nutrient removal and recovery from municipal wastewater, Clean Water, 2020; 7: 1-10.
CrossRef - Oehmen A., Vergel D., Fradinho J., Maria A.M. Reis, João G. Crespo, Velizarov S., Mercury removal from water streams through the ion exchange membrane bioreactor concept, Journal of Hazardous Materials, 2014; 264: 65 - 70.
CrossRef - Malekian R., Abedi-Koupai J., Eslamian S.S., Mousavi S.F., Karim C. Abbaspour, MajidAfyuni, Ion-exchange process for ammonium removal and release using natural iranian zeolite, Applied Clay Science, 2011; 51: 323-329.
CrossRef - Ibrahim M.M., Sayyadi A.S., Application of natural and modified Zeolites in removing heavy metal cations from aqueous media: An overview if including parameters affecting the process, International Journal of Geology, Agriculture and Environmental Sciences, 2015; 3(2): 1-7.
- Vecino X., Reig M., Lopez J., Valderrama C., Gibert O., Cortina Jl., Recovery and separation of valuable metals (copper and zinc) from acidic mine waters by ion-exchange resins, 16th International Conference on Environmental Science and Technology, 2019; 4: 1-2.
- Meng Wang, Karl A. Payne, Tong S., Sarina J. Ergas, Hybrid algal photosynthesis and ion exchange (HAPIX) process for high ammonium strength wastewater treatment, Water Research, 2018; 142: 65-74.
CrossRef - Daochalermwong A., Chanka N., Songsrirote, Dittanet P., Niamnuy C., Seubsai A., Removal of heavy metal ions using modified celluloses prepared from pineapple leaf fiber, ACS Omega, 2020; 5: 5285-5296.
CrossRef - Katsou E., Malamis S., Haralambous K.J., Loizidouv M., Use of ultrafiltration membranes and aluminosilicate minerals for nickel removal from industrial wastewater, Journal of Membrane Science, 2010; 360: 234-249.
CrossRef - Simos Malamis, Evina Kasou, Katerine J. Haralambous, Study of Ni (II), Cu(II), Pb(II), and Zn(II), removal using sludge and minerals followed by MF/UF, Water Air Soil Pollut., 2011; 218: 81-92.
CrossRef - Ngoc T. Bui, Hyungmook Kang, Simon J. Teat , Gregory M. Su, Chih-Wen Pao, Yi-Sheng Liu, Edmond W. Zaia , JinghuaGuo, Jeng-Lung Chen, Katie R. Meihaus, Chaochao Dun, Tracy M. Mattox, Jeffrey R. Long, Fiske P., Kostecki R., Jeffrey J. Urban, A nature inspired hydrogen-bonded supramolecular complex for selective copper in removal from water, Nature Communications, 2020; 11: 3947-3959.
CrossRef - Raghavendra S. Hebbar, Arun M. Isloor, Prabhu B., Inamuddin, Abdullah M. Asiri, Ismail A.F., Removal of metal ions and humic acids throat polyetherimide membrane with grafledbentonite clay, Scientific Reports, 2018; 8: 4665-4681.
CrossRef - Velpula1 S., Umapathy K.S., Thyarla A., Srikanth K., Saraff S., Dairy wastewater treatment by membrane Systems - A review, Int. J. Pure App. Biosci., 2017; 5 (6): 389-395.
CrossRef - Abdullah N., Lau W.L., Yusof N., Jaaafer S., Recent trends of heavy metal removal from water/wastewater by membrane technologies, Journal of Individual Engineering Chemistry, 2019; 46: 17-38.
CrossRef - Ahamefula A. Ahuchaogu, Chukwu O.J., Obike A.I., Chitua E. Igara, Chidi N.I., Echeme J.B.O., Reverse osmosis technology, its applications and nano-enabled membrane, International Journal of Advanced Research in Chemical Science, 2018; 5(2): 20-26.
CrossRef - Pradhan D., Kim D.J., Sukla L.B., Pattanaik A., Lee S.W., Evaluation of molybdenum recovery from sulfur removed spent catalyst using leaching and solvent extraction, Scientific Reports, 2020; 10: 1960-1974.
CrossRef - Yan Li, Limei Yang, ZhengXu, Qi Sun, Separation and recovery of heavy metals from wastewater using synergistic solvent extraction, IOP Conf. Series: Materials Science and Engineering, 2017; 167: 012005-012011.
CrossRef - Katsou E., Malamis S., Loizidou M., Performance of a membrane bioreactor used for the treatment of wastewater contaminated with heavy metals, Bioresource Technology, 2011;102: 4325-4332.
CrossRef - Bazrafshan E., Mohammadi L., Moghaddam A.A, Mahvi A.H., Heavy metals removal from aqueous environments by electrocoagulation process - A systematic review, Journal of Environmental Health Science & Engineering, 2015;13: 74-90.
CrossRef - AL - Shannag M., AL - Qofah Z., Bani-Melhem K., Mohammed RasoolQtaishat, MalekAlkasrawi, Heavy metal ions removal from metal plating wastewater using electrocoagulation: Kinetic study and process performance, Chemical Engineering Journal, 2015; 260: 749-756.
CrossRef - Bhagawan D., Poodari S., Pothuraju T., Srinivasulu D., Shankaraiah G., Rani M.Y., Himabindu V., Vidyavathi S., Effect of operational parameters on heavy metal removal by electrocoagulation, Environ. Sci. Pollut. Res., 2014; 21: 14166-14173.
CrossRef - Antonio G., Merma, Santos B.F., Rego A.S.C., Hacha R.R., Torem M.L., Treatment of oily wastewater from the mining industry using electrocoagulation: Fundamentals and process optimization, Journal of Materials Research and Technology, 2020; 9: 15164-15176.
CrossRef - Bazrafshan E., Moein H., Mostafapour F.K., Nakhaie S., Application of electrocoagulation process for dairy wastewater treatment, Journal of Chemistry, 2013; 39: 1-9.
CrossRef - Rehman A., Kim M., Reverberi A., Fabiano B., Operational parameter influence on heavy metal removal from metal plating wastewater by electrocoagulation process, Chemical Engineering Transactions, 2015; 43: 2251-2256.
CrossRef - Asaithambi P., Beyene d., Aziz A.R.A., Alemayehu E., Removal of pollutants with determination of power consumption from landfill leachate wastewater using an electrocoagulation process: optimization using response surface methodology 9RSM, Applied Water Science, 2018; 8: 69-81.
CrossRef - Naje A.S., Chelliapan S., Zakaria Z., Mohammed A., Ajeel, Alaba P.A., A review of electrocoagulation technology for the treatment of textile wastewater, Rev Chem Eng, 2016; 33(3): 263-292.
- Jagwani M., Er. Dohare D., Electro Coagulation application in water and wastewater treatment: A review, International Journal of Scientific & Engineering Research, 2018; 9(4): 1430-1437
- Butler E., Yung - Tse Hung, Yu-Li Yeh R., AI Ahmad M.S., Electrocoagulation in wastewater treatment, Water, 2011; 3: 495-525.
CrossRef - Laky D., Licsko I., Arsenic removal by ferric-chloride coagulation - effect of phosphate, bicarbonate and silicate, Water Science & Technology, 2011; 64 (5): 1046-1055.
CrossRef - Jing G., Ren S., Gao Y., Sun W., Gao Z., Electrocoagulation: A promising method to treat and reuse mineral processing wastewater with high COD, Water, 2020; 15: 595-607.
CrossRef - Heffron J., Marhefke M., Mayer B.K., Removal of trace metal contaminants from potable water by electrocoagulation, Scientific Reports, 2016; 6: 28478-28487.
CrossRef - Kabuk H.A., Avsar Y., Ilhan F., Ulucan K., Comparison of pH adjustment and electrocoagulation processes on treatability of metal plating wastewater, Separation Science and Technology, 2014; 49: 613 - 618.
CrossRef - Chouhan A., ThakurL K., Patidar K., Varma A.K., A review on removal of heavy metals from water / wastewater by electrocoagulation process, International Research Journal of Engineering and Technology, 2018; 5 (12): 934-944.
- Pang F.M., Teng S.P., Teng T.T., Mohd Omar A.k., Heavy metals removal by hydroxide precipitation and coagulation - flocculation methods from aqueous solutions, Water Qual. Res. J. Can, 2010; 44(2): 174-182.
CrossRef - Xiao Su., Kushima A., Halliday C., Zhou J., Ju Li, Hatton T.A., Electrochemically - mediated selective capture of heavy metal chromium and arsenic oxyanions from water, Nature Communications, 2018; 9: 4701-4710.
CrossRef - Huang T., Dongwei Li., KexiangLi.U., Zhang Y., Heavy metal removal from MSWI fly ash by electrokinetic remediation coupled with a permeable activated charcoal reactive barrier, Scientific Reports, 2015; 5: 15412-15428.
CrossRef






