Chemical Potency of Cobalt Doped Modified Graphite Electrode Prepared by Electrochemical Method and its Application in Degrading Solution of Rhodamine-B dye
Hiremaralli Sathyanarayana Sindhushree1
 , Rayapura Thimmegowda Radhika2
, Rayapura Thimmegowda Radhika2
 and Bellale Marigowda Venkatesha1
*
and Bellale Marigowda Venkatesha1
*

1
Department of Chemistry,
Yuvaraja’s College, University of Mysore,
Mysuru,
Karnataka
India
2
Department of Chemistry,
Maharani’s Science College for women,
Mysuru,
Karnataka
India
Corresponding author Email: bmvenkatesha123@gmail.com
DOI: http://dx.doi.org/10.12944/CWE.19.1.23
Copy the following to cite this article:
Sindhushree H. S, Radhika R. T, Venkatesha B. M, Chemical Potency of Cobalt Doped Modified Graphite Electrode Prepared by Electrochemical Method and its Application in Degrading Solution of Rhodamine-B dye. Curr World Environ 2024;19(1). DOI:http://dx.doi.org/10.12944/CWE.19.1.23
Copy the following to cite this URL:
Sindhushree H. S, Radhika R. T, Venkatesha B. M, Chemical Potency of Cobalt Doped Modified Graphite Electrode Prepared by Electrochemical Method and its Application in Degrading Solution of Rhodamine-B dye. Curr World Environ 2024;19(1).
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2023-11-23 |
|---|---|
| Accepted: | 2024-05-10 |
| Reviewed by: | 
 Balarak Davoud
Balarak Davoud
|
| Second Review by: |

 Peiqiang Li
Peiqiang Li
|
| Final Approval by: | Dr. Gangadhar Andalur |
Introduction
Due to the toxicity of dye which harmful to ecosystem. In many countries throughout the world major contamination has occurred as a result of the widespread discharge of wastewater from industries containing organic dyes onto land and water bodies. The textile, rubber, paper, cosmetic, plastics, leather, pharmacological and foodstuff industries together utilize more than ten thousand effluents, which contributes to the enormous volume of wastewater produced each year. Dyes often consist of relatively large aromatic molecules with several connected rings. The removal of dyes from coloured effluents can be accomplished through a variety of techniques, including precipitation, photodegradation, chemical degradation, biodegradation, and adsorption. As azo dyes are easily converted to potentially dangerous aromatic amine, traditional biological treatment methods under anaerobic circumstances are inefficient for the treatment of synthetic colourants and sewage that contains high salinity colours. Additionally, techniques like adsorption and coagulation are used to clean wastewater dyes, which invariably cause secondary contamination. Traditional treatment methods used in the real world to remove dye effluent fall short of tight discharge requirements. To comply with the discharge standards, an effective method of treating wastewater containing dye is therefore urgently needed.1,2,3 Either a direct oxidation process or an indirect oxidation process leads to the electrochemical breakdown of contaminants. Due to anodic electron transfer process contaminants are first absorbed on the surface of the anode through anodic oxidation method. Potent oxidizing agents like chloroxide and dihydrogen dioxide are produced by electrochemical redox process. The produced oxidant then undergoes an oxidation process, destroying the contaminants in the bulk solution. All of the oxidants are produced on-site and used right away. Direct oxidation and redox pair-mediated electro-oxidation have both been demonstrated in recent years to be competitive solutions for the treatment of textile effluent. 4,5,6,7,8,9,10,11,12 High volumes of highly loaded effluents and waste streams are produced by a variety of industries and other human activities. The difficulty always stems from the absence of a gold standard for the efficient treatment of certain forms of wastewater, regardless of the contamination type. Because highly concentrated waste streams have many characteristics that make them perfect candidates for electrochemical treatment, electrochemistry may find a position in this area. For instance, as the amounts produced are typically modest, electrochemical methods have an advantage because they are typically well-suited to compact decentralised treatment systems.13 For the remediation of organic pollutants, advanced oxidation processes (AOPs) have recently drawn a lot of attention. AOPs work well to completely remove these substances by mineralizing them, enhancing the solution's biodegradability and lowering toxicity. Fenton reaction-based methods have received the most praise among AOPs for their effectiveness in treating resistant organic molecules. But because the Fenton reaction's ferrous regeneration rate was so sluggish, more ferrous ions were needed to remove contaminants efficiently, which in turn increased the amount of sludge produced. With the use of heterogeneous catalysts, chelating agents or by introducing external energy such as electricity as in the electro-Fenton (EF) process which was partially solved, this drawback of the traditional Fenton process was largely overcome.14 Here, we prepared a Co/graphite modified electrode (Co/GME)-based electrochemical technique for the Rhodamine-B dye degradation and investigated its kinetics of degradation.
Experimental
Rhodamine-B (LOBACHEMIE 95% purity) solution [1.0 mM] was prepared. The ALFA AESAR (99.99% purity) graphite rod measuring length of 08.90 cm and width of 0.55 cm in width was used. About 01.60 cm long graphite rod immersed to Rh-B dye solution. The graphite rod was polished by hand with finer grades of emery paper, from 3000 grit to a mirror sheet, in order to prepare it for the experiment. The polished graphite rod was next cleaned with acetone, a 50% dilute hydrochloric acid followed by using ddH2O (double distilled water) before being used meant for more electrochemical deposition. Figure 1 show the electrochemical deposition experimental setup of Cobalt doped graphite modified electrode. CoCl2 (Arora Matthey 99.7%) solution was used to deposit cobalt on a graphite electrode. All of the substances were of recognised purity levels. It consists of a voltage power source and a reaction chamber. Figure 2 show the experimental setup for electrochemical degradation of Rh-B dye in the presence of Cobalt doped GME. Platinum electrode (SKY TECHNOLOGY INDIA PLATINUM ELECTRODE (MODEL NO. STI 519 Pe) 99.9% pure platinum) and Cobalt doped GME were kept at a distance of 2cm apart in Rh-B dye solution. First graphite electrode used as anode in the kinetic runs and in second case, Cobalt doped GME used as anode and electrode of platinum acts as cathode. The experiment was carried for one to two hours under continual stirring of dye solution. For various concentration of Rh-B dye (0.05 millimolar, 0.1 millimolar, 0.15 millimolar and 0.20 millimolar) by applying different applied current output of 2.5mA, 3.5mA, 4.5mA and 5.5mA by using battery (NEULITE INDIA) and Rheostat (INSIFINDIA). pH and chemical oxygen demand values were measured for the pre degradation and post degradation of dye. Spectrometer (ELICO SL 171) was used to measure the percentage transmittance of degradation of Rh-B. By varying 3 temperatures degradation of Rh-B was studied using graphite electrode and Cobalt modified graphite electrode one is room temperature at 298 K other two temperatures were maintained using thermostat(308 K) and ice cold water bath thermostat (293 K).
 | Figure 1: Experimental setup of electrochemical deposition of Cobalt doped GME
|
 | Figure 2: Electrochemical experimental setup for the Rhodamine – B dye degradation
|
Result and Discussion
Electrochemical degradation of Rhodamine-B dye using graphite electrode
Effect of Dye concentration
Degradation of different concentration of dye was examined by electrochemical method at constant current of 3.5mA. Using a spectrophotometer, the color change of dye solution indicates the change in [Rh-B]. The linear behavior of velocity of dye degradation is shown in a plot of log %T v/s time, showing that first-order kinetics governs the removal of Rhodamine-B dye up to 65% of degradation reaction. Table 2 shows that values for the rate constants with variation of concentration of Rh-B. In the presence of higher concentration of dye solution the degradation rate decreased. This is due to the accumulation of very lean layer of Rh-B electrolyte at the outer most layer of graphite anode as a result of this the concentration of .OH free radicals decreased, which slows down current flow across the electrode-solution interface. Figure 3(A & B) and table 2 shows the measured pH and chemical oxygen demand values of dye solution before and after electrolysis of dye. After degradation, the pH value reveals a slightly increase in the pH. From the pHzpc graphs for graphite electrode shown in figure 4 indicates the surface of adsorbent is positively charged. In the presence of Co metal ion it shows more pH after degradation process therefore degradation efficiency is more than initial but less than degradation by Co/GME.
Table 2: Concentration effect and COD measurements on degradation rate of Rh-B
[Rh-B] millimolar | k 105 per second | Variation of pH | Chemical Oxygen Demand value in milligrams per litre | ||
Initial pH | Final pH | Prior to degradation | Later degradation | ||
0.05 | 5.066 | 6.65 | 6.79 | 446 | 16 |
0.10 | 4.222 | 6.98 | 7.23 | 816 | 16 |
0.15 | 1.343 | 7.16 | 7.28 | 992 | 32 |
0.20 | 1.036 | 7.25 | 7.38 | 1224 | 48 |
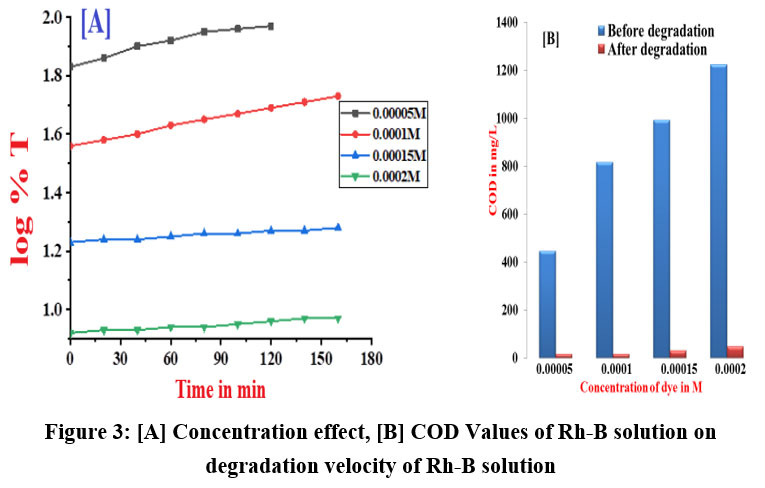 | Figure 3: [A] Concentration effect, [B] COD Values of Rh-B solution on degradation velocity of Rh-B solution
|
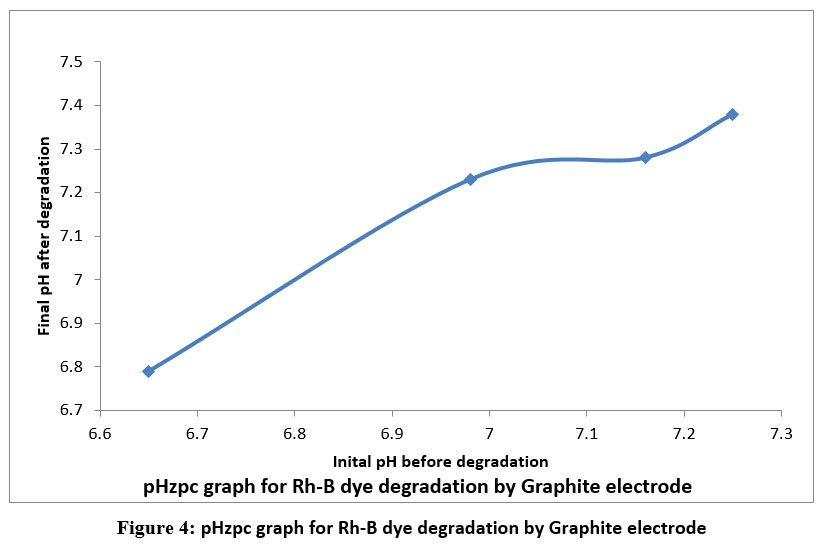 | Figure 4: pHzpc graph for Rh-B dye degradation by Graphite electrode
|
Effect of applied current
In assessing the impact of current density, the experiment was run with four distinct currents that range from 2.5 mA - 5.5 mA while maintaining the same [Rh-B]. Increase in applied current increases the velocity of degradation process because it raises concentration of .OH radicals and oxidising intermediates. Figure 5 (A & B) and table 3 shows the rate constant and chemical oxygen demand readings of various current density of Rh-B electrolytic solution were measured for prior and later the electrochemical decolorization process.
Table 3: Current effect and measurement of COD on degradation rate of Rh-B solution
Current in milliamp | k 105 per second | Chemical Oxygen demand value in milligrams per litre | |
Prior to degradation | Later degradation | ||
2.5 | 2.686 | 816 | 16 |
3.5 | 4.222 | 816 | 32 |
4.5 | 7.292 | 816 | 16 |
5.5 | 8.444 | 816 | 32 |
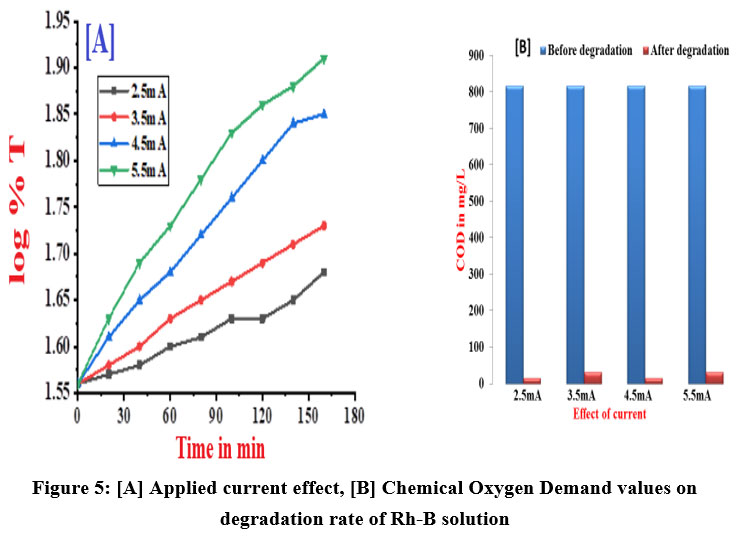 | Figure 5: [A] Applied current effect, [B] Chemical Oxygen Demand values on degradation rate of Rh-B solution
|
Effect of temperature
Table 4 shows the velocity constant and measured Chemical oxygen demand values of the decolorization of Rh-B electrolytic solution at three distinct temperatures shown in figure 6 (A & B). Low temperatures have been found to have a negligible impact on the rate of color degradation. However, higher temperatures have a more significant effect on the reaction. An increase in temperature raises the velocity of diffusion and electro chemical degradation. Table 5 shows the calculated thermodynamic parameters.
Table 4: Temperature effect and COD values of degradation velocity of Rh-B solution
Temp. in Kelvin
| k 104 per second | Chemical Oxygen demand value in milligrams per litre | |
Prior to degradation | Later degradation | ||
293 | 3.454 | 816 | 32 |
298 | 4.222 | 816 | 16 |
308 | 6.141 | 816 | 16 |
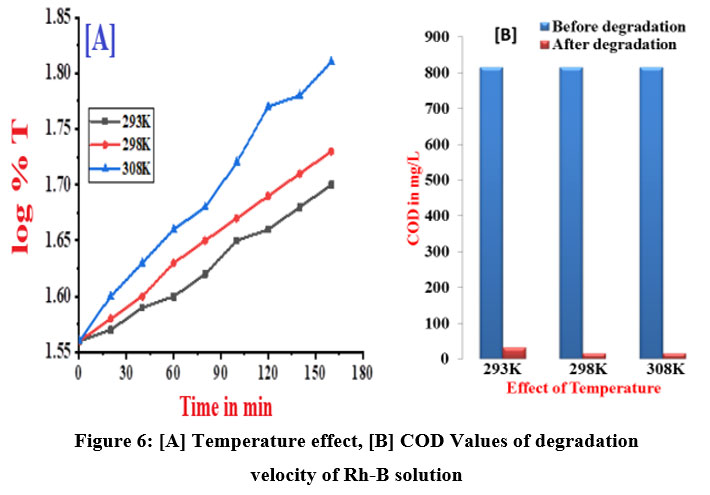 | Figure 6: [A] Temperature effect, [B] COD Values of degradation velocity of Rh-B solution
|
Table 5: Thermodynamic parameters for Rh-B solution by graphite electrode.
Temperature in Kelvin | H# kiloJoule per mol | S# Joule per Kelvin per mol | G# kiloJoule per mol | Ea kiloJoule per mol |
293 | 63.86 | -114.23 | 97.86 |
66.46 |
298 | 63.96 | -114.69 | 98.32 | |
303 | 64.02 | -114.90 | 98.10 |
Degradation of Rhodamine-B by Cobalt doped GME
Effect of dye concentration
During electrochemical reaction by using four various concentration of Rh-B solution was taken, at a constant current density of 3.5mA was employed. The colour change was utilised to measure the variation in Rhodamine-B concentration using a spectrophotometer. Electro degradation of Rh-B electrolytic solution follows kinetics of first order reaction, as shown by the linear behavior in a graph plot of log %T v/s time in minutes and table 6 shows the rate constant values. Rate of reaction decreased as the [Rh-B] was raised. The rate of degradation of Rhodamine-B using Co/GME as anode was higher than when only graphite electrode used as the anode. After degradation, the pH value increases slightly, towards an alkaline pH. The velocity constant and calculated COD values for the different [Rh-B] solutions were listed in table 6 and shown in figure 7 (A and B). From the pHzpc graph of the degradation of Rh-B solution by Cobalt doped graphite electrode shown in figure 8 that the surface of adsorbent is positively charged. In the presence of Co metal ion it shows more pH after degradation process because of the production of .OH free radical therefore degradation efficiency is more by Co/GME.
Table 6: Concentration effect and measurement of COD on degradation rate of Rh-B using Cobalt doped GME
[Rh-B] millimolar | k 104 per second | Variation of pH | Chemical Oxygen demand value in milligrams per litre | ||
Initial pH value | Final pH value | Prior to degradation | Later degradation | ||
0.05 | 2.341 | 06.71 | 06.82 | 446 | 16.0 |
0.10 | 2.149 | 06.98 | 07.29 | 816 | 16.0 |
0.15 | 1.919 | 07.16 | 07.27 | 992 | 48.0 |
0.20 | 1.458 | 07.19 | 07.27 | 1224 | 64.0 |
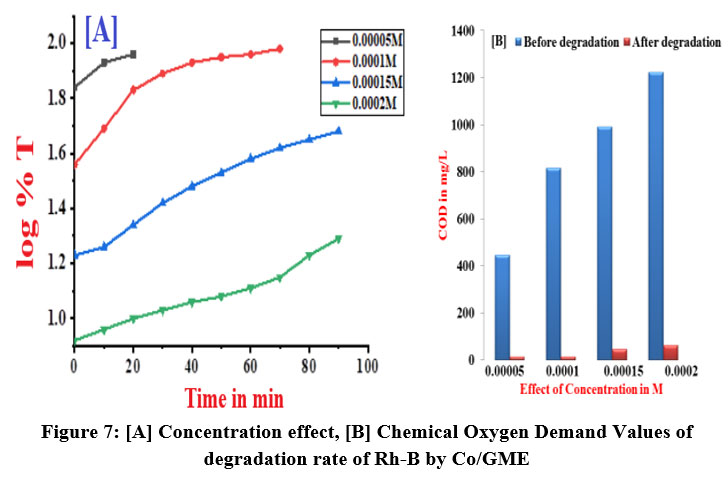 | Figure 7: [A] Concentration effect, [B] Chemical Oxygen Demand Values of degradation rate of Rh-B by Co/GME
|
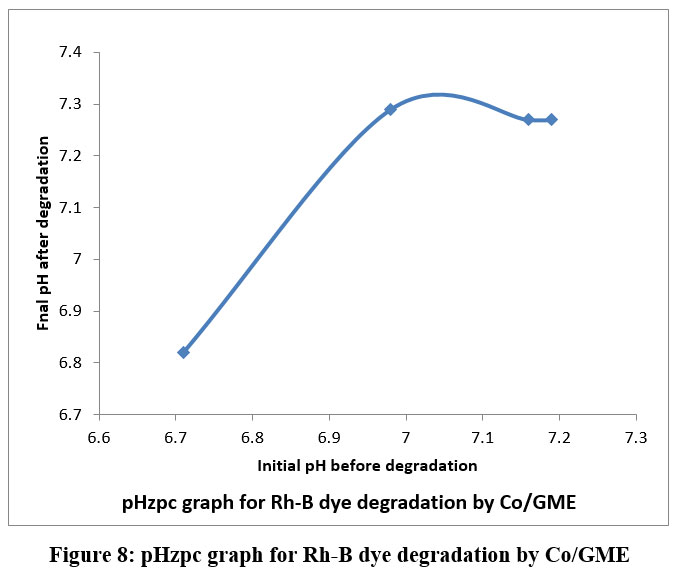 | Figure 8: pHzpc graph for Rh-B dye degradation by Co/GME
|
Effect of current
By increasing applied current density the rate of reaction increases at fixed [Rh-B]. The range of the applied current is 2.5mA to 5.5mA. Degradation rate of Rh-B solution increases with rise in applied current since it promotes the formation of oxidising intermediates and .OH radicals. Due to Cobalt catalytic activity, the rate of degradation was higher than it would have been for a graphite electrode. Figure 9 (A & B) and Table 7 shows data of the rate constants and the measurement of COD values for the conditions before and after degradation of Rh-B dye solution.
Table 7: Current effect and measurement of Chemical oxygen demand values on degradation rate of Rh-B solution using Cobalt doped GME
Current in milliamp | k 104 per second | Chemical Oxygen demand value in milligrams per litre | |
Prior to degradation | Later degradation | ||
2.5 | 1.420 | 816 | 48 |
3.5 | 2.149 | 816 | 32 |
4.5 | 2.533 | 816 | 16 |
5.5 | 3.876 | 816 | 32 |
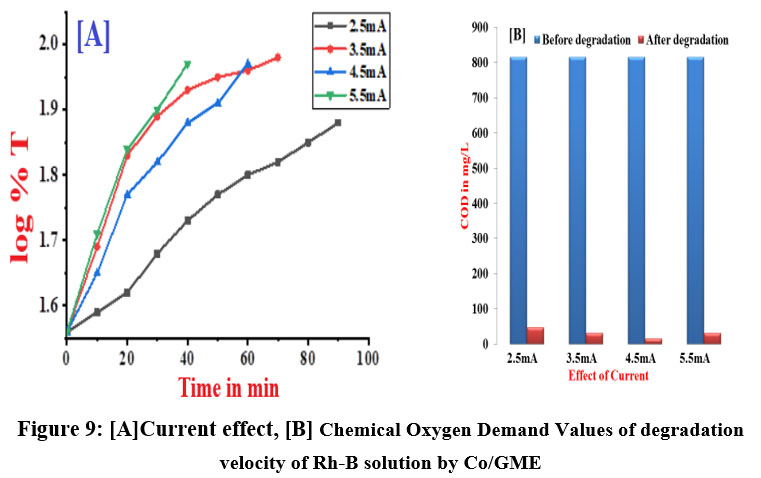 | Figure 9: [A]Current effect, [B] Chemical Oxygen Demand Values of degradation velocity of Rh-B solution by Co/GME
|
Temperature Effect
In order to evaluate the temperature effect ,studies have been done at three temperatures. The raise in the temperature accelerates the process, and it has been noted that at low temperatures, the degradation rate is not significant. However at higher temperatures, the rate of reaction strongly impacted. When compared to graphite electrodes, Co/GME had a higher degrading efficiency. The rate constants and COD values for both the initial state and the degraded state are shown in Table 8 and Figure 10 (A and B). An increase in temperature increase the rate of reaction since the diffusion rate rises with temperature. The calculated thermodynamic parameters values of Rh-B dye degradation using Co doped Graphite modified electrode has been reported in table 9. At different temperature energy of activation and other thermodynamic parameter values is less in the degradation of dye by Co/GME and hence degradation rate increases related to graphite anode degradation of dye. As temperature increases, kinetic energy increases effective collision increases and energy of activation decreases. Therefore rate of electrochemical degradation reaction increases.
Table 8: Temperature effect and measurement of Chemical oxygen demand values on degradation rate of Rh-B solution using Cobalt doped graphite modified electrode
Temp. in Kelvin | k 104 per second | Chemical oxygen demand values in milligram per litre | |
Prior to degradation | Later degradation | ||
293 | 1.535 | 816 | 32 |
298 | 2.149 | 816 | 16 |
308 | 3.799 | 816 | 16 |
Table 9: Thermodynamic parameters for Rh-B degradation by Cobalt doped graphite electrode
Temperature in Kelvin | H# kiloJoule per mol | S# kiloJoule per mol | G# kiloJoule per mol | Ea kiloJoule per mol |
293 | 36.39 | -185.96 | 95.8 |
40.78 |
298 | 36.28 | -186.74 | 94.24 | |
303 | 35.18 | -186.86 | 94.32 |
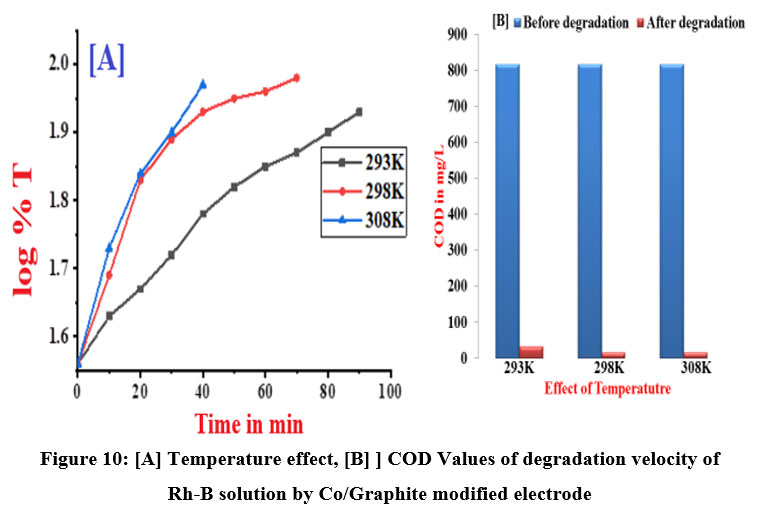 | Figure 10: [A] Temperature effect, [B] ] COD Values of degradation velocity of Rh-B solution by Co/Graphite modified electrode
|
Production of .OH Free radicals
By anodic oxidation process, Rhodamine-B solutions have been degraded. During electrochemical process, while using graphite electrode as the anode and Pt as the cathode, .OH free radicals are produced by electrolysis and these radicals acts as an intermediate at the anodic surface of a high voltage of O2- by the oxidation of water.
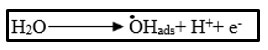
Rhodamine-B degrades as a result of electrochemical reduction and oxidation that occur in sequence.15,16 Rhodamine-B has a high affinity for graphite electrodes, which implies that this will negatively impact electrode performance through competing of interactions and adsorption of electron transfer increases the rate of reaction. Rhodamine-B reacts with the powerful oxidant .OH free radical, sequential redox reaction of Rhodamine-B with graphite and platinum electrodes to produce CO2, H2O, and inorganic salts (shown in scheme 1) such as bromides till the point of their complete mineralization takes place. It has been found that the platinum electrode has more O2- over-voltage (+0.77 V), which results in more oxidant .OH radical being produced. When the dye material is degraded, a colorless dye solution is recovered, showing the absence of metal oxides. It indicates the electro degradation of dye takes place as a result of oxidising intermediates that are produced. Taking into an account, the benefits of graphite electrodes (GE), including their commercial availability, low cost and ease of modification.17,18,19,20,21 Wastewater remediation can be accomplished using the current technique.
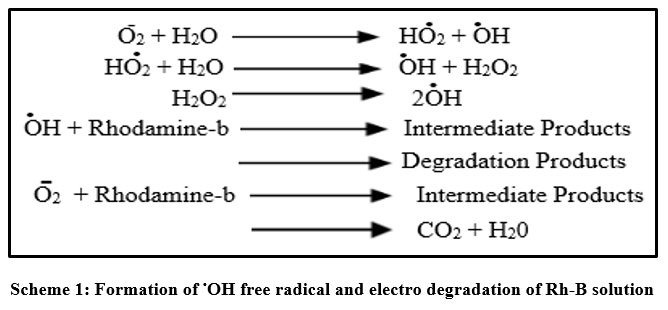 | Scheme 1: Formation of .OH free radical and electro degradation of Rh-B solution
|
Effect of treatment duration on COD
It has been found that the dye degradation rate affects COD. From the initial COD of 446 mg/L, a 95% decrease in COD has been achieved during this trial resulting in 40 mg/L of COD. Its levels were within the permitted range of 250 mg per litre after degradation. Figure 11 shows a graph that plots COD reduction versus treatment time. Measurements were made during the impact of current density, [dye], and COD. The COD level was reduced by more than 90% in each of these situations. For the anodic oxidation of the Rhodamine-B, the values of the COD were utilised to calculate the instantaneous current efficiency (ICE) from the equation 1.
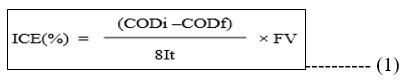
Here, ‘F’ represents Faraday constant, ‘V’ represents volume of electrolytic solution, ‘I’ represents the applied current and ‘t’ is time interval and ‘8’ is equivalent mass of oxygen. Chemical oxygen demands before and after degradation is designated as CODi and CODf, respectively. The ICE statistics in Tables (10, 11) shows that process efficiency is linearly related to ICE and that process efficiency for Co/GME anodes is higher than for GE anodes.
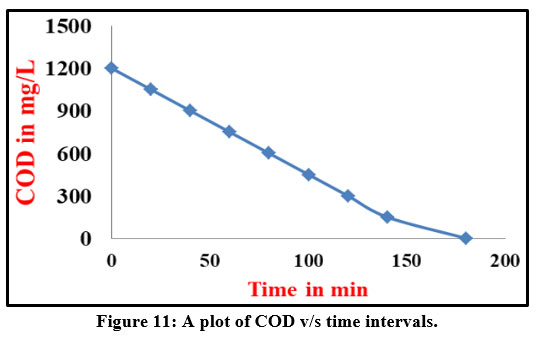 | Figure 11: A plot of COD v/s time intervals.
|
Table 10: ICE Values for Graphite Electrode
[Rh-B] 10-4M
| Variables | ICE |
0.5 | 165.48 | |
1.0 | 159.82 | |
1.5 | 128.36 | |
2.0 | 114.23 | |
Current in mA | 2.5 | 172.32 |
3.5 | 159.82 | |
4.5 | 127.21 | |
5.5 | 121.32 | |
Temperature in K | 293 | 103.04 |
298 | 159.82 | |
308 | 182.47 |
Table 11: ICE Values for Co/GME
[Rh-B] 10-4M
| Variables | ICE |
0.5 | 616.72 | |
1.0 | 392.62 | |
1.5 | 321.67 | |
2.0 | 283.42 | |
Current in mA | 2.5 | 352.16 |
3.5 | 392.62 | |
4.5 | 436.72 | |
5.5 | 486.90 | |
Temperature in K | 293 | 279.79 |
298 | 392.62 | |
308 | 426.88 |
Kinetics of Rhodamine-B Degradation
The rate of degradation depends on the quantity of Surface Active Sites [S], current [I] and [dyes] in the lack of electrolytes like Hydrochloric acid or sodium hydroxide. At constant [S], the rate equation for dye degradation is shown below.
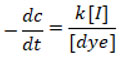
The rate constant for Rhodamine-B solution throughout degradation reaction it was found by a plot of log of percentage transmittance versus time indicates kinetics study follows first order rate of reaction when measured by a spectrophotometer at wavelength hmax 545 nanometer. Up to 60 % of the degradation process has been achieved by a straight line, however after that, a linearity deviation was observed.
Reusability of Cobalt doped graphite modified electrode
Reuse of Co/graphite-modified electrode was investigated. The modified graphite electrode was properly cleaned with ddH2O following the degradation of the dye, and it was then repurposed for the degradation using a new dye solution. The degradation investigation revealed that the reuse of the electrode with graphite modification for the dye solution to degrade had slightly lower efficiency. While reuse of Co/GME for the degradation of standard dye solution shows less adsorption. Multiple use of Co/GME for the degradation of dye shows 2% less efficiency than we used it freshly modified graphite electrode.
Ultra Violet – Visible spectral analysis
Figure 10 (A) and (B) shows the UV-visible spectrum of Rh-B dye before and after degradation. After the degrading procedure, a wide range of spectral peak at 550 nanometer were not appeared.
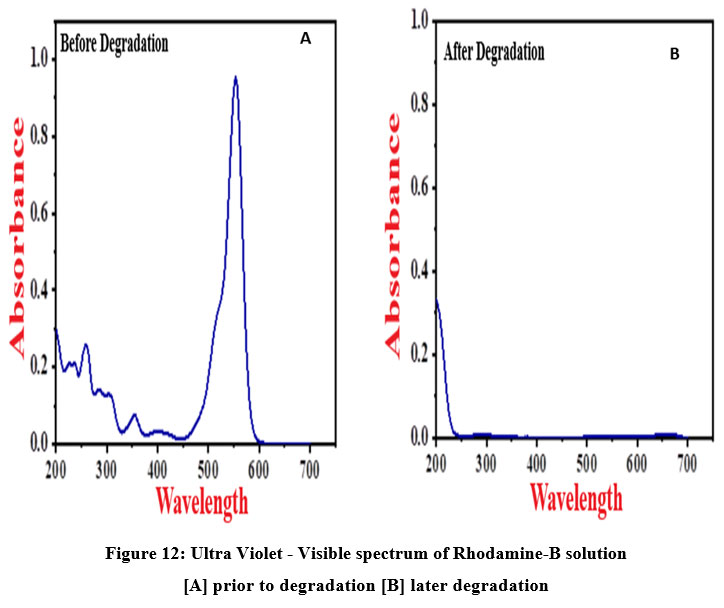 | Figure 12: Ultra Violet - Visible spectrum of Rhodamine-B solution [A] prior to degradation [B] later degradation
|
FE - SEM and EDX
The difference between the Cobalt (Co) coated graphite electrode and the bare graphite electrode is determined using FE-SEM. Figures 13(A) and (B) displays Scanning electron micrograph and EDX analysis of graphite anode. The layering and homogeneity of the graphite were seen in various diameters in the microscope. From the figure 13(A) it is evident that the presence of graphite flakes with the size varied from 10 – 50 µm. The only carbon elements in the EDX profile revealed that there was pure graphite present Figure. 13(B) and Table 13 show the EDX quantitative result for graphite anode.
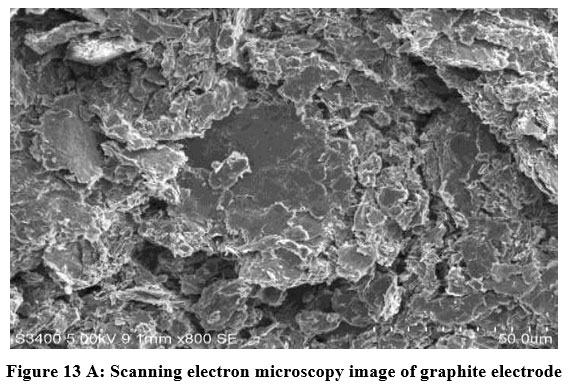 | Figure 13 A: Scanning electron microscopy image of graphite electrode
|
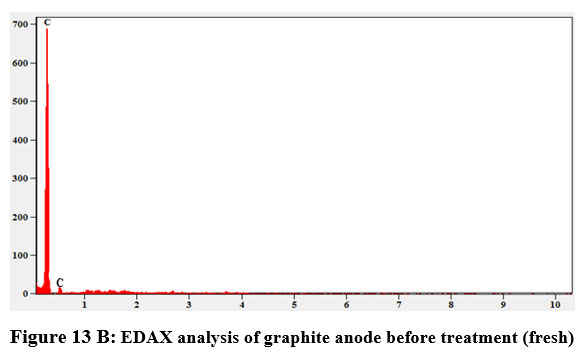 | Figure 13 B: EDAX analysis of graphite anode before treatment (fresh)
|
Table 12: EDX spectral quantifiable data for graphite electrode
Element | Weight % | Atom % |
Carbon K | 100.0 | 100.0 |
Carbon K | 0.00 | 0.00 |
Total | 100.0 | 100.0 |
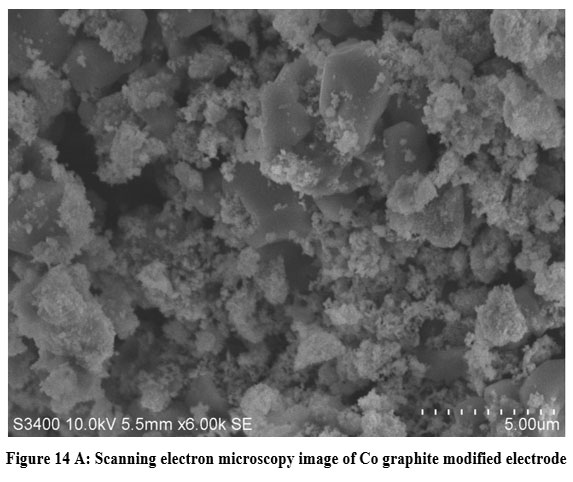 | Figure 14 A: Scanning electron microscopy image of Co graphite modified electrode
|
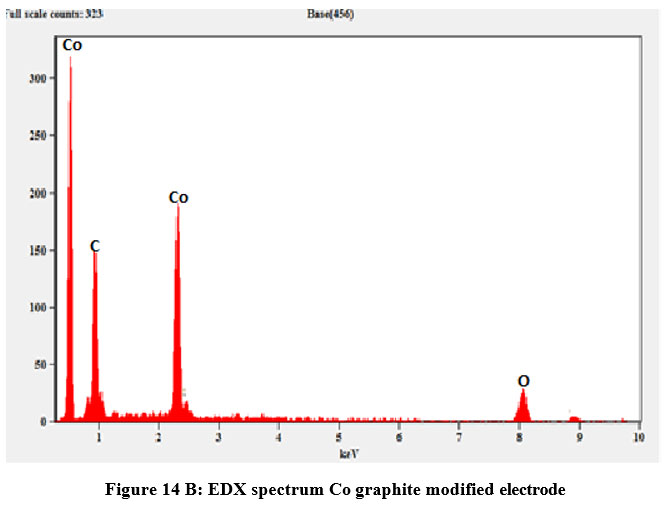 | Figure 14 B: EDX spectrum Co graphite modified electrode
|
Table 13: EDX quantifiable data for Cobalt doped graphite electrode
Element | Weight in Percentage | Error Weight in Percentage | Atom in Percentage |
O K | 46.03 | ± 1.39 | 58.97 |
O L | --- | --- | --- |
Co K | 53.97 | ± 5.96 | 41.03 |
C L | --- | --- | --- |
total | 100 | --- | 100 |
SEM and EDX profiles of cobalt thin layer on the rod of graphite has been displayed in figure 14 A and B. In spectrum of Scanning Electron Micrograph, normal graphite rods and cobalt encapsulated graphite rods may be plainly distinguished from one another. Furthermore, on the surface heterogeneous block dots were seen, indicating that the graphite rod may have been doped with cobalt and Co is deposited within the graphite rod. We were able to see cobalt peaks in different oxidation phases as well as the graphite carbon peak on the EDX map Figure.14 (B) and Table 13 shows the EDX quantitative result for Cobalt doped graphite modified Electrode.
Conclusion
Electrochemical oxidation using a graphite electrode and Co/GME study found that the dye chromophore groups that are frequently present in industrial effluents could be removed with minimum energy usage and graphite electrode recycling. This method can be used to clean up organic and coloured wastewater since it makes use of a graphite electrode and Co/GME. The degradation of Rh-B dye and COD removal rates by the Co/GME electrode were higher than those by the graphite electrode.
Acknowledgements
The authors are grateful to late Prof.S.Ananda, former Professor and chairman, UGC – BSR faculty fellow, DOS in Chemistry, Manasagangothri, University of Mysore, Mysuru, for his keen encouragement and timely guidance, and also authors are acknowledges Yuvaraja’s college, IOE,UPE & CPEPA, University of Mysore.
Funding Sources
The authors received no financial support for the research, authorship and publication of this article.
Conflict of Interest
The authors declare no conflict of interest.
Author’s Contribution Statement
H. S. Sindhu shree: Conceptualization, writing- original draft, Data Curation, Formal analysis, Visualization, Methodology.
R. T. Radhika: Investigation, Validation, Visualization, Methodology.
B. M. Venkatesha: Supervision, Validation, Writing- review and editing.
Ethics Approval Statement
The authors declare no ethical conflicts of interest regarding the publication of this paper.
Data Availability Statement
The authours declares that the data used in the study are original and findings in ours experimental investigation.
The manuscript incorporates all datasets produced or examined throughout this research study.
References
- Rathinam R, Govindaraj K, Vijayakumar, Pattabhi. Decolourization of Rhodamine B from aqueous solution by electrochemical oxidation using graphite electrodes. Desalination and Water Treatment.2015;01-07.
CrossRef - Fang Han, Venkata Subba Rao Kambala, Madapusi Srinivasan, Dharmarajan Rajarathnam, Ravi Naidu.Tailored titanium dioxide photocatalysts for the degradation of organic dyes in wastewater treatment: A review. Applied catalysis A: General.2009;315:25-40.
CrossRef - Mondal S. Methods of dye removal from dye house effluent-An overview. Environmental Eng. Sci.2008;25(3) 383-396.
CrossRef - Mohan N, Balasubramanian N, Basha C.A. Electrochemical oxidation of textile wastewater and its reuse.Journal of Hazardous Materials.2007;147(1-2):644-651.
CrossRef - Ahlawat R, Srivastava V.C, Mall I.D, Sinha S.Investigation of the electrochemical treatment of cotton blue dye solutions using aluminium electrodes.Clean.2008;36(10-11):863-869.
CrossRef - Bhatnagar R, Joshi H, Mall I.D, Srivastava V.C. Electrochemical treatment of acrylic dye bearing textile wastewater: optimization of operating parameters. Desalination Water Treat. 2013;52(1-3):111–122.
CrossRef - Mondal B, Srivastava V.C, Mall I.D.Electrochemical treatment of textile printing wastewater by stainless steel electrodes: multiple response optimization and residue analysis. Journal of Environ. Sci. Health Pt. A.2012;47:2040–2051.
CrossRef - Govindaraj M, Rathinam R, Sukumar C, Uthayasankar M, Pattabhi S. Electrochemical oxidation of bisphenol-A from aqueous solution using graphite electrodes. Environmental Technology.2013;34(4): 503-511.
CrossRef - Zhang X.D, Li W.S, Huang Y.Y, Peng H.Y. Promotion of Oxygen Reduction Reaction on vitreous Carbon Electrode by DTAB. Acta. Physico-Chimic.Sinica.2008;24(04): 691-694.
CrossRef - Martinez-Huitle C.A, Quiroz M.A, Cominellis C, Ferro S, Battisti A.D. Electrochemical incineration of chloranilic acid using Ti/IrO2, Pb/PbO2 and Si/BDD electrodes. Electrochimica Acta.2004;50(04): 949-956.
CrossRef - Raju G. B, Karuppiah M.T, Latha S.S, Parvathy S, Prabhakar S.Treatment of wastewater from synthetic textile industry by electrocoagulation-electrooxidation. Chemical Engineering Journal.2008;144(1):51-58.
CrossRef - Orlando G.R, Emmanuel M, Hugo O.V, Olivier L. Electrochemical treatment of highly concentrated waste water: A review of experimental and modelling approaches from lab-to full-scale. Critical Reviews in Environmental Science and Technology.2022;52(2): 240-309.
CrossRef - Puthiya V. N, Soliu O. G, Martinez-Huitle C. A, Emmanuel M, Hugo O. V, Clement T, Minghua Z, Oturan M. A. Recent advances in electro-Fenton process and its emerging applications. Critical Reviews in Environmental Science and Technology.2023;53(8): 887-913.
CrossRef - Wang A, Qu J, Ru J, Liu H, Ge J. Mineralization of an azo dye Acid Red 14 by electro-Fenton’s reagent using an activated carbon fiber cathode. Dyes and Pigments.2005;65(3): 227-233.
CrossRef - Ammar S, Abdelhedi R, Flox C, Arias C, Brillas E. Electrochemical degradation of the Indigo carmine at boron-doped diamond anode for wastewaters remediation. Environmental Chemistry Letters.2006;4(4): 229-233.
CrossRef - Sowbhagya, Ananda S, Rakesh. Electrochemical degradation of Indigocarmine dye at Ru-doped platinum anode in aqueous solution. International Journal of Applied Chemistry.2012;8(2): 141-152.
- Tao Xu, Lanyere Fu, Huiying Lu, Mengyum Zhang. Electrochemical oxidation degradation of Rhodamine B dye on boron-doped diamond electrode: Input mode of power attenuation. Journal of cleaner production. 2023;401(1): 136794.
CrossRef - Qizhou Dai, Lei Jiang, Xubiao Luo. Electrochemical Oxidation of Rhodamine B: Optimization and Degradation Mechanism. International Journal of Electrochemical Science. 2017;12(5): 4265-4276.
CrossRef - Vasudevan S, Lakshmi J, Sozhan G. Effects of alternating and direct current in electrocoagulation process on the removal of cadmium from water. Journal of Hazardous Materials.2011;192(1): 26-34.
CrossRef - Ignasi sires, Mehmet A. Oturan, Marco Panizza. Electrochemical Advanced Oxidation processes: Today and Tommorow. A Review. Environmental Science Pollutant. 2014:(14):8336-67
CrossRef - Three-Dimensional particle electrode system treatment of organic wastewater: A general review based on patents. Journal of cleaner production. Journal of cleaner production. 2021(308).
CrossRef






