Influence of Integrated Approach of Azotobacter and Nitrogen Fertilizer on Various Morpho-Physiological and Biochemical Parameters of Brassica Oleracea L. var. capitata
Corresponding author Email: psaffeullah.139@gmail.com
DOI: http://dx.doi.org/10.12944/CWE.16.Special-Issue1.06
Copy the following to cite this article:
Saffeullah P, Nabi N, Umar S, Influence of Integrated Approach of Azotobacter and Nitrogen Fertilizer on Various Morpho-Physiological and Biochemical Parameters of Brassica Oleracea L. var. Capitata. Curr World Environ 2021; SI1. DOI:http://dx.doi.org/10.12944/CWE.16.Special-Issue1.06
Copy the following to cite this URL:
Saffeullah P, Nabi N, Umar S, Influence of Integrated Approach of Azotobacter and Nitrogen Fertilizer on Various Morpho-Physiological and Biochemical Parameters of Brassica Oleracea L. var. Capitata. Curr World Environ 2021; SI1. Available From : https://bit.ly/3cqManj
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 31-08-2020 |
|---|---|
| Accepted: | 01-02-2021 |
| Reviewed by: | 
 Prashant Sharma
Prashant Sharma
|
| Second Review by: |

 Vyacheslav M Semenov
Vyacheslav M Semenov
|
| Final Approval by: | Dr. Monika J. Kulshrestha |
Introduction
Food securityis one of the critical challenges we are facing today.1Nevertheless, agricultural lands have been overexploited for centuries; and itsfallouts are soil degradation, erosion, and increased greenhouse gas emissions, due to higher dependency on agronomic inputs.2Some of the proposed options for improving soil fertility include organic amendments, no-till farming, use of bio-fertilizers and integrated nutrient management system.3 World agricultural output has tremendously increased since 1950’s by the effort of scientific community to ensure food security. But these modern methods of agriculture have led to deleterious effects on our environment.
About 80-90 million tons (MT) of nitrogen (N) fertilizers are supplied to soil throughout the world each year.4To maintain the sustainability of agricultural production is itself a great challenge. However during the last few years, bio-fertilizers have appeared as an imperative part of integrated nutrient management system and seems a promising approach for increasing crop yield and free nutrient supply to soil.5 In recent years, there has been anabundantattention in establishing associations amongstcrops and various N2-fixing microbes.6Azotobacter(AzB) can be acrucialalternate of syntheticN fertilizers because it deliversN in the form of ammonia (NH3), nitrate (NO3-) and amino acids, which could be potentialreplacements of inorganic nitrogen source.7It also aids to sustain the plant growth and yield even in situation of low phosphate content in soil, as well as assists in uptake of nutrients which enablesimproved utilization of plant root exudates.8
The genus Azotobacter, belonging to family Azotobacteriaceae, is the key group of heterotrophic non-symbiotic N-fixing bacteria. The non-symbiotic free living Azotobacteris mostlyrelated with N fixation in plant rhizosphere9and is capable of fixing at least 10 mg N per g of carbohydrate.10,11Azotobacter spp. are most specifically noted for their N fixing ability; although they have also been noted for their capability to provide different growth hormones (like IAA, gibberllins and cytokinins), vitamins and siderophores.12When Azotobacter is applied to seeds, seed germination is enhanced to a substantial extent besides improving growth.13
Brassica oleracea L.var.capitata,a member of the genus Brassica(family Brassicaceae),commonly known as cabbage, is consumed either raw as salad or processed in different ways such as boiled or fermented.14Cabbage has extensive use in traditional medicine due to its antioxidant, antibacterial and anti-inflammatory properties.15It is also used in easing of symptoms linkedwith numerous gastrointestinal disorders as well as in treatment of minor cuts and wounds and mastitis.15 The seeds are anthelmintic, diuretic, laxative and stomachic. They have been used in the treatment of gout.16Thephytochemicals present in cabbage canalleviate and stabilize the body’s antioxidant and detoxification mechanisms that eradicate cancer-causing substance.17,18Since the indiscriminate and excessive use of N fertilizers is often practiced in cabbage farms, in pursuit of maximum yield. So this study was designed to test this alternative fertilizer (Azotobacter) to lower the use of synthetic N. Keeping in view the importance of cabbage, the present study was conducted to elucidate the effect of N and AzB treatments on cabbage (Brassica oleracea L. var. capitata).
Materials and Methods
Authentic seeds of Brassica oleraceaL. var. capitata [genotypeEarly golden acre (L) and Pusa drumhead (H)] were procured from IARI, New Delhi. Seeds were surface sterilized by 0.1% mercuric chloride solution for 3 min, then washed with sterilized distilled water (5-times) and air-dried at an ambient temperature of 27°C in the laboratory. Following treatments of N (supplied as urea) and AzB were applied at the time of sapling transplantation as seedling treatment after 3-4 weeks of seed germination (Table 1).
Table 1: Various Treatments of N and Azotobacter(AzB) (alone and in combination) applied to Low and High NE Genotypes used in the Present Study.
|
Name assigned |
Treatment applied |
|
T0 |
Control |
|
T1 |
60 kgha-1 N |
|
T2 |
60 kgha-1 N + AzB |
|
T3 |
120 kgha-1 N |
|
T4 |
120 kgha-1 N + AzB |
|
T5 |
180 kgha-1 N |
|
T6 |
180 kgha-1 N + AzB |
|
T7 |
AzB alone |
The Saplings were Dipped in Liquid Containing
Azotobacterchroococcum(AzB:DW-1:5) before transplantation. The experiment was performed under natural field conditions in prepared beds in herbal garden of Jamia Hamdard, New Delhi, India. There were 8 beds comprising 3 replicates for each treatment and each genotype. Experiments were arranged in randomized block design with three replicates. The soil is classified as FluvicCambisol (Humic) according to the classification of the Food and Agriculture Organization (FAO Rome).Some of the soil characteristics and climatic conditions of the experimental area are given in Table 2 and Fig. 1, respectively.Plants were sampled at three growth stages and harvested after 90 days of treatment and the results were recorded during the time frame of experiments.
Table 2: Some Physico-Chemical Characteristics of Pre-Treated Soil (0-30 cm),of the Study Area. Data Represents mean±standard Deviations (n=3).
|
Soil Characteristic |
Value |
Unit |
|
pH |
7.21±0.4 |
- |
|
Electrical Conductivity |
0.15±0.06 |
dS m-1 |
|
Soil organic carbon |
1.62±0.2 |
g kg-1 |
|
Cation exchange capacity |
20.1±0.9 |
cmol kg-1 |
|
Available N |
0.43±0.04 |
g kg-1 |
|
Available P |
0.07±0.01 |
g kg-1 |
|
Available K |
0.26±0.08 |
g kg-1 |
|
C/N ratio |
3.76±0.12 |
- |
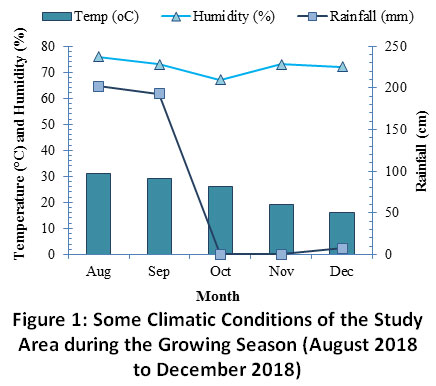 |
Figure 1: Some Climatic Conditions of the Study Area during the Growing Season (August 2018 to December 2018) Click here to view Figure |
Parameters Studied
Growth Characteristics
Plants were dig up cautiously and cleaned by distilled water. At 12-week stage, plant and root length was noted down. Shoot fresh weight (g) was measured by electronic balance. Plant samples were dried in an oven at 75°C for 3 days. After three days, shoot and root dry weight (g) was recorded again. Dry weights of the entire plant, shoot and root were determined by drying in an oven at 68°C to a constant weight. The number of leaves was manually calculated at the end of the study; leaf area was also measured.
Estimation of Total Chlorophyll
Photosynthetic pigments, the chlorophyll a, b and total chlorophyll contents in the leaf were estimated in fresh samples by the method of Hiscox and Israelstam19using dimethyl sulphoxide(DMSO). Vials containing 100 mg of chopped leaves in 10 mLDMSO were covered with aluminum foil and kept in oven at 65°C for an hour. The reaction mixture was taken in a cuvette and finally absorbance was read at 480, 510, 645, 663 nm by using UV-Vis spectrophotometer (Systronics, India).
Nitrate Reductase(NR) Activity
Nitrate reductase(NR) activity was assessed by the intact tissue assay method of Jaworski20whichtakes into account the reduction of NO3- to nitrite (NO2-). Leaf material (250 mg) was suspended in screw capped vial containing 2.5 mL each of phosphate buffer (0.1 M, pH 7.5), potassium nitrate solution (0.2 M) and iso-propanol (5%), and 2 drops of chloramphenicol (0.5%). The vials were incubated at 30°C in the dark for about 2 hours. NR activity in the medium was determined by taking 0.4 mLof incubated solution and 0.3 mL each of sulphanilamide (1% in 3N HCI) and NEDD (0.02%). After 20 minutes, the solution was diluted with 4 mL of distilled water to make the final volume upto 5 mL and its optical density measured at 540 nm. A standard curve was plotted using varying concentrations of potassium nitrite and used for calculations.
Total Soluble Protein Content
The total soluble protein content of different sample was estimated following the method of Bradford.21Chopped fresh leaf tissues (0.5 g) were crushed in 1.5 mL of phosphate buffer (0.1 M) at 4°C. The homogenate was centrifuged at 5000 rpm for 20 min at 4°C. An identicalvolume of 10% TCA (pre-chilled)was added to 0.5 mL of the supernatant, which was centrifuged again at 3500 rpm for 20 min. Further, 0.2 mLaliquot was added to 1 mL of Bradford’s reagent. The absorbance was noted at 595 nm on a Beckman spectrophotometer (DU 640, Fullerton, USA). The protein content was expressed in mg g-1f.w.
Estimation of Sugar
This method given by Dey22 was used for estimating sugar content. 0.1 g of fresh sample was weighted to which 10 ml of ethanol was added and the mixture was incubated at 60°C for 1 hour. After that, 1 mLof phenol was added to 1 mL of aliquot and was vortexed. Then, 5 mL of sulphuric acid was added to the reaction mixture which was later cooled in air. Absorbance was measured at 485 nm.
Statistical Analysis
Data represents mean±standard deviation (SD). Values are means of three replicates and the presented mean values were analyzed by SPSS software (version 22) for one-way ANOVA. Significant differences were estimated using Duncan’s Multiple Range Test (DMRT) at p≤ 0.05.
Results
Growth Attributes
The plant height, number of leavesand leaf area showed significant variations between the genotypes and different treatments. The variations in the morphological features of two genotypes are given in Table 3.
A significant difference was found in shoot fresh and dry weight at varying N concentrations. The shoot fresh weight was found to increase significantly with an upsurge in N fertilization and was maximum at 180 kgha-1. it was also found to decrease with the increasing age of the plant. So, fresh weight decreased in all the treatments at post-flowering stages (Table 4). This rise in shoot fresh weight may be attributed the ability of the plant to assimilate the given nitrogen into proteins and other necessary metabolites. The fall in shoot fresh weight with increasing age may be due to the transition of the plant from vegetative phase to flowering phase of its life cycle, whereby a plant tries to concentrate and protect its progeny rather than accumulating biomass.
A similar trend was followed by the root fresh weight. As we increased the dosage of N the fresh weight was found to increase significantly. The increasing root fresh weight is an evidence to the fact that as plant is supplied more N, it tries to develop its root systems extensively in order to absorb more and more nutrients especially N.
Table 3: Changes in Morphological Characteristics of Two Genotypes of Brassica Oleracea L. var. as Affected by Varying Applications of N and AzB at Harvest Stage.
|
Treatment |
Plant height (cm) |
Leaf number |
Leaf area (cm2) |
|
Control L |
8.6±0.36ee) |
5±0.82c |
34.4±2.36e |
|
Control H |
11.2±1.21 D |
7±0.91 D |
42.32±2.03 F |
|
60 Na)Lb) |
12.66±0.35 d |
6±0.39 c |
51.02±3.11 d |
|
60 N Hc) |
13.26±0.52 C |
9±0.65 C |
54.06±2.36 E |
|
60 N + AzBd)L |
8.9±0.84 e |
7±0.81 b |
81.6±3.08 c |
|
60 N + AzBH |
13.6±0.65 C |
9±0.36 C |
56.18±3.26 E |
|
120 N L |
14.2±0.39 c |
7±0.85 b |
117.3±3.44 b |
|
120 N H |
18.2±0.82 B |
11±0.32 B |
76.22±4.11 D |
|
120 N + AzBL |
14.6±0.84 c |
8±0.25 b |
95.12±4.21 c |
|
120 N + AzBH |
18.9±0.91 B |
10±0.44 B |
88.16±4.05 C |
|
180 N L |
16.5±0.92 b |
7±0.36 b |
81.34±3.85 c |
|
180 N H |
21.0±0.66 A |
12±0.32 A |
133.32±3.66 B |
|
180 N + AzBL |
17.2±0.45 a |
10±0.14 a |
102.85±3.25 a |
|
180 N + AzBH |
23.4±0.54 A |
13±0.35 A |
216.25±4.85 A |
|
AzB alone L |
13.1±0.36 d |
7±0.22 b |
83.43±8.22 c |
|
AzB alone H |
21.2±0.25 A |
8±0.35 C |
149.34±5.26 B |
a)N supplied as kgha-1
b) L – genotype low (Early golden acre)
c)H – genotype high (Pusa drumhead)
d) AzB- Azotobacter
e)Different smallcase and uppercase letters denote significant difference (p≤0.05 by DMRT) between the treatments in G-low and G-high, respectively
Table 4: Changes in Fresh and Dry Weight Content of Root and Shoot Two Genotypes of Brassica Oleracea L. var. Capitataas affected by Varying Applications of N and AzB at Harvest Stage.
|
Genotype x Treatment |
Root fresh weight (g) |
Root dry weight (g) |
Shoot fresh weight (g) |
Shoot dry weight (g) |
|
Control L |
7.38±0.66de) |
0.114±0.003e |
178.20±3.99e |
23.124±0.108c |
|
Control H |
6.53±0.83 C |
0.265±0.007 D |
348.90±7.51 E |
95.139±0.079 D |
|
60 Na)Lb) |
11.80±0.87 b |
0.150±0.013 d |
538.50±5.48 d |
82.020±0.195 b |
|
60 N Hc) |
8.00±0.36 A |
0.451±0.037 C |
1162.83±9.42 D |
123.141±0.109 C |
|
60 N + AzBd) L |
9.10±0.62 c |
0.170±0.005 c |
735.67±5.44 c |
85.204±0.114 b |
|
60 N + AzB H |
6.30±0.50 B |
0.458±0.026 C |
1362.47±3.91 C |
126.272±0.138 C |
|
120 N L |
9.60±0.44 c |
0.181±0.005 c |
834.20±9.33 b |
94.133±0.101 b |
|
120 N H |
7.77±0.74 B |
0.714±0.021 B |
1742.33±11.91 B |
142.184±0.116 B |
|
120 N + AzB L |
11.80±0.85 b |
0.190±0.004 c |
882.50±4.14 b |
93.336±0.076 b |
|
120 N + AzB H |
9.17±0.87 A |
0.724±0.011 B |
1823.50±12.73 B |
158.206±0.035 B |
|
180 N L |
8.33±1.17 c |
0.515±0.004 a |
957.33±8.04 a |
121.166±0.149 a |
|
180 N H |
10.87±1.55 A |
0.942±0.029 A |
1982.80±13.91 B |
188.483±0.707 A |
|
180 N + AzB L |
14.23±1.83 a |
0.541±0.015 a |
1033.53±4.28 a |
127.396±0.609 a |
|
180 N + AzB H |
9.87±0.70 A |
0.948±0.016 A |
2302.40±8.31 A |
198.398±0.118 A |
|
AzB alone L |
11.77±0.76 b |
0.218±0.011 b |
495.97±17.55 d |
22.647±0.064 c |
|
AzB alone H |
8.87±0.75 A |
0.313±0.010 C |
1105.17±14.71 D |
92.695±0.053 D |
a)N supplied as kgha-1
b) L – genotype low (Early golden acre)
c)H – genotype high (Pusa drumhead)
d) AzB- Azotobacter
e)Different smallcase and uppercase letters denote significant difference (p≤0.05 by DMRT) between the treatments in G-low and G-high, respectively.
Chlorophyll Content
Chlorophyll content was found to increase with increasing N inputs (Fig. 2). Plants grown in N with inoculation by AzB were found to have greater chlorophyll contents than their counterparts. Genotype high (H) was found to have more chlorophyll content than genotype low (L) under all the treatments. A significant difference wasobserved between pre and post flowering stages of growth. At the time of pre flowering stage the chlorophyll content was found to be increased but later it was found to be decreased at flowering stages. Plants take up more nitrogen at this time so as to form many metabolites. The significant increase in chlorophyll content was due to the ability of the plant to assimilate more and more of the available N into proteins and chlorophyll molecules.
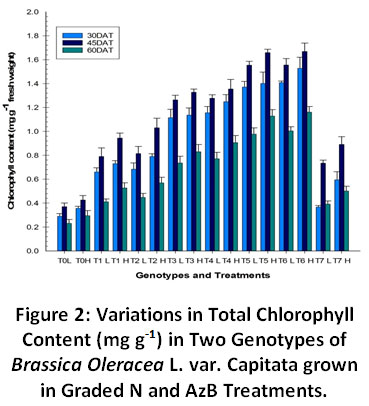 |
Figure 2: Variations in Total Chlorophyll Content (mg g-1) in Two Genotypes of Brassica Oleracea L. var. Capitata grown in Graded N and AzB Treatments. Click here to view Figure |
Nitrate Reductase(NR) Activity
NR activity depicted a significant (p≤ 0.05) variation in leaves of Brassica oleracea grown under combined treatments of N and AzB at 30, 45 and 60 days after treatment (DAT) (Fig. 3). The leaf blade depicted the highest level of NR activity at 30 DAT followed by 45 DAT and 60 DAT. The NR activity was found to increase as we increased the application rate of N upto120 kg ha-1 but further increase in N application didn’t comply with increasing NR activity rather the enzyme became saturable and NR activity was found to decrease slightly in all plant parts at 180 kgha-1. It may also be due to the reason that there is an upper limit of N utilization and consequently plant performance. Moreover, there is an upper limit to the levels of N metabolizing enzymes that the plant can accommodate. Consequently, the plant continued the nitrate uptake due to its ample availability in the soil but was not able to assimilate it. This often leads to nitrate accumulation in plants.
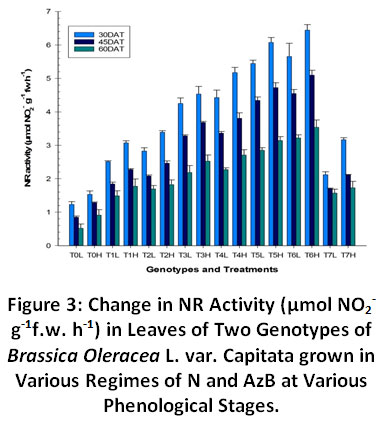 |
Figure 3: Change in NR Activity (µmol NO2- g-1f.w. h-1) in Leaves of Two Genotypes of Brassica Oleracea L. var. Capitata grown in Various Regimes of N and AzB at Various Phenological Stages. Click here to view Figure |
Protein Content
The protein content increased significantly (p≤0.05) as we increased the N concentration in the soil medium (Fig. 4). Plants grown under higher N regimes were found to assimilate more of the nitrate and ammonia into the proteins and hence maximal protein content was found in their leaf tissue. Protein content was found to increase significantly in plants grown in N supplemented with AzB in all the treatments. The protein content varied in the plants at their different phenological ages. It was found to be highest at rosette stage but was found to decrease in harvest stages of plant growth.
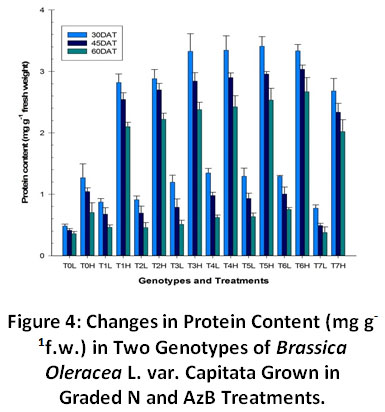 |
Figure 4: Changes in Protein Content (mg g-1f.w.) in Two Genotypes of Brassica Oleracea L. var. Capitata Grown in Graded N and AzB Treatments. Click here to view Figure |
Soluble Sugar Content
A significant effect of nitrogen fertilization and AzB inoculation on soluble sugar concentrations in cabbage was revealed (Fig. 5). The highest concentration of soluble sugars was found in cabbage receiving N and AzB. N fertilization applied on the rows of plants significantly increased concentration of soluble sugars in cabbage in relation to control plants. Highest sugar content was found at 180 kgha-1 N +AzB. The increase in soluble sugars is evident from the fact that as chlorophyll increases the sugar content also increases in the plant tissue. However, the sugar content was lower in all the treatments at Harvest stages due to shift of the plant growth from vegetative to flowering period. Genotype H was found to have increasing amounts of sugar content than L in all the treatments.
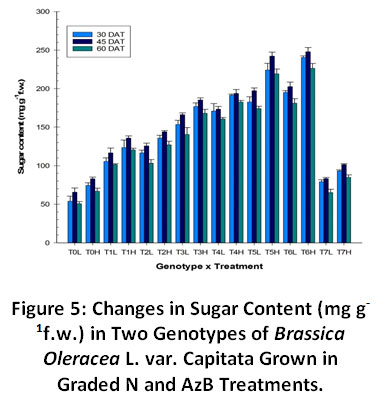 |
Figure 5: Changes in Sugar Content (mg g-1f.w.) in Two Genotypes of Brassica Oleracea L. var. Capitata Grown in Graded N and AzB Treatments. Click here to view Figure |
Total Phenol Content
Total phenol content was found to increase with increasing N fertilizers (Fig. 6). Increase was more prominent in plants grown in combined application of N and AzB. Genotype H was found to have more phenolic content than genotype L in all the treatments. Phenolic content initially increased from initial stage to rosette stage but showed a decline at harvest stage. The treatment containing plants supplemented with AzBalone showed a significant increase in phenol content than control plants.
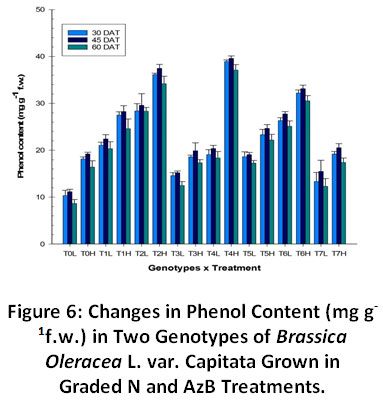 |
Figure 6: Changes in Phenol Content (mg g-1f.w.) in Two Genotypes of Brassica Oleracea L. var. Capitata Grown in Graded N and AzB Treatments. Click here to view Figure |
Discussion
Nitrogen is often regardedas one of the limiting factors for plant growth in agricultural systems.23Because of the key role of N in plant metabolism; one would assume plants to utilize the entireN available to them for biomass production. But, although additionof fertilizers generally results in improved yield, the effectiveness of the uptake and assimilation declines with the increasing level of fertilization.24Therefore, large amounts of fertilizers are left unused, leading to environmental and ecological hazards (like nitrate leaching into groundwater and nitrate accumulation in leafy vegetables) as well as economic loss.25 Thus application of excess N fertilizers, a common agricultural practice, leads to nitrate accumulation in leafy vegetables in one hand and nitrate leaching into the ground water on other, both culminating into undesired environmental effects.26
Application of chemical fertilizer primarily targeted at enhancing its yield has not only interfered with non-targeted microbial activities, but has also affected the quality of cabbage. To reduce such deleterious effects, microbial inoculant particularly free living nitrogen fixers have been tried as an alternative or as a supplement to chemical nitrogen.27 Apart from reducing the ill effects of chemical fertilizer, microbial inoculant could also maintain the quality of the cabbage and reduce the cost of cultivation. Azotobacter, a free living aerobic microbe has been utilized for enhancing the performance of crops by supplying them additional N requisite for their growth and productivity. Application of Azotobacter would reduce the dependence on inorganic sources of nitrogen. The experiment was designed to find out the effect of various levels of nitrogen and Azotobacter with its interaction effects to find out the best combination of different treatments to achieve maximum economic returns on growth, yield and quality of cabbage.
In this study, fresh and dry weights were found to increase significantly with an increase in N inputs. The increase was more pronounced in plants grown in integrated system of N and Azotobacter. Plants grown in Azotobacteralone were found to have fresh and dry weights significantly higher than control. The increase in fresh and dry weights may be attributed to the increasing amount of N availability which further complements plant growth. These outcomesare in accord with the opinions of Razaqet al.28and Kızılkaya29, who have revealedadvantageous effects of bio-fertilizers on crop growth due to deliverance of growth promoting substances.
The improvement in yield characteristics as a result of using Azotobacter alone or in combination with N fertilizer may be due to the enhancementin vegetative growth (plant height, fresh and dry weight,leaf number, and total chlorophyll) in the treated plots. Kızılkaya28found a similar pattern in potato plants, reporting an increase in yield due to Azotobactertreatment. This enhancement may beattributable to increased production of plant hormones like auxins(IAA) and gibberellins together with the vitamins (Biotin, folic acid and vitamin B groups). The processes ofphotosynthesis andproteinsynthesis were enhanced by the treatments that subsequentlycaused an increase in productivity per unit area. These outcomes are consistent with those of Kahlel30by growing potato crop in AzotobacterandN treatments.
Our results found a significant increase in NR activity with an increase in N content at rosette stage. NR activity was found to be lower in petiole followed by lower leaf and middle leaf and highest in upper leaf. Similar findings were reported by Sareeret al.31 in AndrographisandSaffeullah et al.32in B. oleracea. Nitrate content also followed the reverse trend showing maximum accumulation in petiole followed by lower leaf and middle leaf and minimum accumulation in upper leaf. Our results are in agreement in Mazaharet al.33
Sugar content showed an increase with increasing N treatments, highest achieved in combination with Azotobacter. Similar findings were reported in cabbage by Sadyet al.34
The consumption of food having higher phenolic content can check chronic diseases associated to oxidative stress in the human body.35,36Like other phytochemicals, thelevel of phenolic compounds in crops, can be influenced by various factors such as cultivar used,agro-climatic features, and storage environments.34,37 Our results suggest that the total phenol content increased with increasing N concentrations. Results were more pronounced in test plants treated with N + Azotobacter. The increase was more prominent at rosette stage and showed a decline at harvest stage. Genotypepusa drumhead was found to contain more phenolic compounds than pusa golden acre. The level of phenolic compounds in vegetables is genotypespecific;though, it is greatly altered by the method and rate of N fertilization.38
Conclusion
This study concludes that Azotobacter seedling treatments enhance the growth of cabbage genotypes. Azotobacter alone or in combination with N fertilizer significantly enhances chlorophyll content, nitrate reductase activity, protein and phenol content in cabbage plants and considerably improves the yield of the cabbage genotypes. Thus, the use of Azotobacter in integrated nutrient management systems is recommended whichmay help in the expansion of organic farming practices and will substantiallylessen the cost of fertilizer application and environmental menacescaused due to largerdependency on chemical fertilizers.
Funding Source
The first author is highly thankful to University Grants Commission (UGC), India for financial assistance under grant no. 172000514.
Acknowledgements
The authors would like to thank JamiaHamdard, New Delhi, for granting the Ph.D.Research work.The Department of Botany, JamiaHamdard, and Department of Botany, University of Kashmir, is highly appreciated for allowing the laboratory work. The author is profoundly grateful to University Grants Commission (UGC), India, for financial assistance.
Conflict of Interest
The authors declare that they have no conflicts of interest to declare.
References
- Zhang F., Cui Z., Fan M., Zhang W., Chen X., Jiang R. Integrated soil–crop system management: reducing environmental risk while increasing crop productivity and improving nutrient use efficiency in China. Journal of Environmental Quality 2011; 40(4):1051-1057.
CrossRef - Aguilera E., Lassaletta L., Gattinger A., Gimeno B. S. Managing soil carbon for climate change mitigation and adaptation in Mediterranean cropping systems: a meta-analysis. Agriculture, Ecosystems & Environment 2013; 168:25-36.
CrossRef - Lal. Restoring soil quality to mitigate soil degradation. Sustainability 2015; 7(5):5875-5895.
CrossRef - Moose S., Below, F. E. Biotechnology approaches to improving maize nitrogen use efficiency. In: Molecular genetic approaches to maize improvement. Springer: Berlin, Heidelberg, 2009; pp. 65-77.
CrossRef - Bhardwaj D., Ansari M. W., Sahoo R. K., Tuteja N. Biofertilizers function as key player in sustainable agriculture by improving soil fertility, plant tolerance and crop productivity. Microbial Cell Factories 2014; 13(1):1-10.
CrossRef - Alhia B. M. H. The Effect of Azotobacterchrococcum as nitrogen biofertilizer on the growth and yield of Cucumissativus. 2010.
- Ghimire R., Khanal A., Kandel B. P., Chhetri L. B. Effect of nitrogen and pre-planting treatment of seedling with Azotobacter on growth and productivity of broccoli (Brassica oleracea var. italica). World Scientific News 2018;109:267-273.
- Yadav S., Singh K., Chandra R. Plant Growth–Promoting. Microbes for Sustainable Development and Bioremediation 2019;207.
CrossRef - Fatima P., Mishra A., Om H., Saha B., Kumar P. Free Living Nitrogen Fixation and Their Response to Agricultural Crops. Biofertilizers and Biopesticides in Sustainable Agriculture2019;173.
CrossRef - Sahoo R. K., Ansari M. W., Dangar T. K., Mohanty S., Tuteja N. Phenotypic and molecular characterisation of efficient nitrogen-fixing Azotobacter strains from rice fields for crop improvement. Protoplasma 2014; 251(3):511-523.
CrossRef - Becking J. H. The family azotobacteraceae In:The prokaryotes. Springer: New York,1992; pp. 3144-3170.
CrossRef - Noumavo P. A., Agbodjato N. A., Baba-Moussa F., Adjanohoun A., Baba-Moussa L. Plant growth promoting rhizobacteria: Beneficial effects for healthy and sustainable agriculture. African Journal of Biotechnology 2016; 15(27):1452-1463.
CrossRef - Jnawali A. D., Ojha R. B., Marahatta S. Role of Azotobacter in soil fertility and sustainability–A Review. Adv Plants Agric Res. 2015; 2(6):1-5.
- Patra J. K., Das G., Paramithiotis S., Shin H. S. Kimchi and other widely consumed traditional fermented foods of Korea: a review. Frontiers in Microbiology 2016; 7:1493.
CrossRef - Kapusta-Duch J., Kopec A., Piatkowska E., Borczak B., Leszczynska T. The beneficial effects of Brassica vegetables on human health. RocznikiPaństwowegoZakładuHigieny 2012; 63(4).
- Jaradat N. Ethnopharmacological survey of natural products in Palestine. 2005.
- Ezena G. N. Exploiting the Insecticidal Potential of the Invasive Siam Weed, ChromolaenaOdorata L.(Asteraceae) In the Management of Major Pests of Cabbage, Brassica OleraceaVarCapitata and Their Natural Enemies for Enhanced Yield in the Moist Semi-Deciduous Agro-Ecological Zone of Ghana (Doctoral dissertation, University of Ghana). 2015.
- Singh J., Upadhyay A. K., Bahadur A., Singh B., Singh K. P., Rai M. Antioxidant phytochemicals in cabbage (Brassica oleracea L. var. capitata). ScientiaHorticulturae 2006; 108(3):233-237.
CrossRef - Hiscox J. D.,Israelstam G. F. A method for the extraction of chlorophyll from leaf tissue without maceration. Canadian Journal of Botany1979;57(12):1332-1334.
CrossRef - Jawaroski E. G. Nitrate reductase assay in intact plant tissues. Biochemical and Biophysical Research Communications1971; 43:1274-1279.
CrossRef - Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Analytical Biochemistry1976; 72:248-254.
CrossRef - Dey P.M. Methods in plant biochemistry. Carbohydrates, Vol 2. Academic Press:London, 1990; pp. 675.
- Follett R. F. Transformation and transport processes of nitrogen in agricultural systems. In: Nitrogen in the Environment. Academic Press, 2008; pp. 19-50.
CrossRef - Li T., Zhang W., Yin J., Chadwick D., Norse D., Lu Y., Dou Z. Enhancedâ€efficiency fertilizers are not a panacea for resolving the nitrogen problem. Global Change Biology 2018; 24(2):511-521.
CrossRef - Wang Z. H., Li S. X. Nitrate N loss by leaching and surface runoff in agricultural land: A global issue (a review). In Advances in Agronomy. Academic Press, 2019; Vol. 156, pp. 159-217.
CrossRef - Ahmed M., Rauf M., Mukhtar Z., Saeed N. A. Excessive use of nitrogenous fertilizers: an unawareness causing serious threats to environment and human health. Environmental Science and Pollution Research 2017; 24(35):26983-26987.
CrossRef - Zaidi A., Khan M. S., Saif S., Rizvi A., Ahmed B., Shahid M. Role of nitrogen-fixing plant growth-promoting rhizobacteria in sustainable production of vegetables: current perspective. In: Microbial strategies for vegetable production Springer: Cham. 2017; pp. 49-79.
CrossRef - Razaq M, Zhang P, Shen H.Salahuddin. Influence of nitrogen and phosphorous on the growth and root morphology of Acer mono. PLoS ONE 2017; 12(2):e0171321.
CrossRef - Kızılkaya R. Yield response and nitrogen concentrations of spring wheat (Triticumaestivum) inoculated with Azotobacterchroococcum strains. Ecological Engineering 2008; 33(2):150-156.
CrossRef - Kahlel A. M. S. Effect of organic fertilizer and dry bread yeast on growth and yield of potato (Solanumtuberosum L.). J Agric Food Tech. 2015; 5(1):5-11.
- Sareer O., Ahmad S., Umar S. Andrographispaniculata: a critical appraisal of extraction, isolation and quantification of andrographolide and other active constituents. Natural Product Research 2014; 28(23):2081-2101.
CrossRef - Saffeullah P., Liaqat S., Nabi N., Kain T. A., Siddiqi T. O., Ahmad S., Umar S. Amenability of indigenous genotypes of cabbage to scavenge and accumulate nitrogen: importance of staggered application and root morphology. Journal of Plant Biology 2020. https://doi.org/10.1007/s12374-020-09264-4.
CrossRef - Mazahar S., Sareer O., Umar S., Iqbal M. Nitrate accumulation pattern in Brassica under nitrogen treatments. Brazilian Journal of Botany 2015; 38(3):479-486.
CrossRef - Sady W., Wojciechowska R., Rozek S. The effect of form and placement of N on yield and nitrate content of white cabbage. In: International Conference on Environmental Problems Associated with Nitrogen Fertilisation of Field Grown Vegetable Crops.1999; 563:123-128.
CrossRef - Ismail A., Marjan Z. M., FoongC. W. Total antioxidant activity and phenolic content in selected vegetables. Food Chemistry 2004; 87(4):581-586.
CrossRef - Podsędek A. Natural antioxidants and antioxidant capacity of Brassica vegetables: A review. LWT-Food Science and Technology 2007; 40(1):1-11.
CrossRef - Smoleń S., Sady W. The influence of nitrogen fertilization and Pentakeep V application on contents of nitrates in carrot. ActaHort et Regiotec. 2009; 12:221-223.
- Stumpf B., Yan F.,Honermeier B. Influence of nitrogen fertilizer on yield and phenolic compounds in wheat grains (Triticumaestivum L. ssp. aestivum). Journal of Plant Nutrition and Soil Science2018; 182(1).
CrossRef






