Metal Induced Risk of Diabetes Mellitus Due to Toxicological Effects of Mercury: Influence of Environmental Threats
1
Department of Zoology,
Barasat Govt. College,
Kolkata,
West Bengal
India
Corresponding author Email: guriasrikanta@gmail.com
DOI: http://dx.doi.org/10.12944/CWE.15.3.11
Copy the following to cite this article:
Guria S. Metal Induced Risk of Diabetes Mellitus Due to Toxicological Effects of Mercury: Influence of Environmental Threats. Curr World Environ 2020;15(3). DOI:http://dx.doi.org/10.12944/CWE.15.3.11
Copy the following to cite this URL:
Guria S. Metal Induced Risk of Diabetes Mellitus Due to Toxicological Effects of Mercury: Influence of Environmental Threats. Curr World Environ 2020;15(3). Available from: https://bit.ly/36DZXCm
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 26-05-2020 |
|---|---|
| Accepted: | 30-10-2020 |
| Reviewed by: | 
 Kasun Samarasiri
Kasun Samarasiri
|
| Second Review by: |

 Chuck Chuan NG
Chuck Chuan NG
|
| Final Approval by: | Dr Gopal Krishan |
Mercury is an environmental pollutant which produces health hazard (Marx, 2002; Ratcliffe et al., 1996). Its application is found in agriculture as fungicide, in medicine as topical antiseptic, disinfectant as well as amalgam fillings in dentistry (ATSDR, 1999).
Patients with Minamata disease (methylmercury poisoning) in Japan showed incidence of diabetes mellitus (DM) (Takeuchi and Eto, 1997; Uchino et al., 1995). The study of Shigenaga (1976) showed that repeated treatment of rats with methylmercury (MeHg) induced diabetes mellitus (DM). Recently, Chen et al, (2006 and 2010) stated that mercuric compounds influence pancreatic β-cell dysfunction. Toxic metals due to pollution and industrialization like lead (Pb), nickel (Ni), cadmium (Cd), arsenic (As) and mercury (Hg) are associated with alteration of glucose homeostasis and cause progress of diabetes (Khan and Awan, 2014).
Toxic metal-induced oxidative stress may decrease activity of insulin gene promoter and insulin mRNA expression in pancreatic islet β-cells and, thus, alter the glucose regulations (Zheng et al., 2018). Mercury and arsenic can induce many disorders including DM by oxidative stress leading to apoptosis (Chen et al., 2009; Jomova et al., 2011).
This present study will explain the toxicological effects of mercury (II) chloride (HgCl2) in the pancreatic islets as well as liver tissues of rat to explain metal-induced diabetes mellitus (DM).
Materials and Methods
This study will elucidate the toxicological effects of mercury (II) chloride (HgCl2) in the pancreatic islets as well as liver tissues of rat by following methods.
Animals and housing
The study was carried out on two groups of albino rats weighing between 100 to 120 g (total 14 rats, each group contained 7 animals).
Animals in all groups were fed ad libitum and allowed free access to water and daily diet. All animals were acclimatized in laboratory condition and received human care.
Rats were divided into control group and their respective treated group (n=7/group). Rats (treated group) were injected with HgCl2 (5 mg/kg/day) for 2 to 3 successive weeks (Chen et al., 2012) and control rats only received normal daily diet and water.
Histological investigation
Paraffin tissue sections of liver and pancreas were stained with haematoxylin and eosin (H&E) and periodic acid/Schiff (PAS) and examined.
Pancreatic cells isolation or extraction
Pancreas was removed using the forceps and mashed through the cell strainer into the petridish containing (0•1) Μ phosphate buffer saline (pH 7•2) in presence of trypsin- EDTA and tryton X 100. Cell suspension was subjected for centrifugation at 800xg for 3 minutes. Supernatant was discarded and pellet was resuspended in PBS. Cell suspension was taken for study.
Trypan blue dye exclusion test
Cells were treated with trypan blue dye solution for 5 minutes and observed under a light microscope and mortality index was calculated.

Glucose Tolerance Test (GTT)
Blood was collected from the tail veins of control and mercury treated rats for glucose tolerance test (GTT) by glucometer (Accu Chek Active Strip 25S) (Chakrabarti et al., 2007; Guria et al., 2012 and 2014; Guria, 2017 and 2018).
Results and Discussion
Histopathological findings of pancreas and analysis of pancreatic cells
Histopathology of islets of Langerhan's of pancreas of control animal revealed normal architecture with compact arrangement of cells. The islets seemed lightly stained than the surrounding acinar cells (Fig. 1A). Photomicrograph of histology of treated pancreas exhibited the disruption of islets, disorientation of cells and disruption of connective tissue septa (Fig. 1B).
Photographs of control giemsa stained pancreatic cells showed intact nuclei and membrane (Fig. 1C). In treated group pancreatic cells were found to be pyknotic and cellular death was confirmed by membrane rupture, necrosis and nuclear degeneration (Fig. 1D). Trypan blue (TB) positive response was noticed in majority of treated pancreatic cells (Fig. 1E).
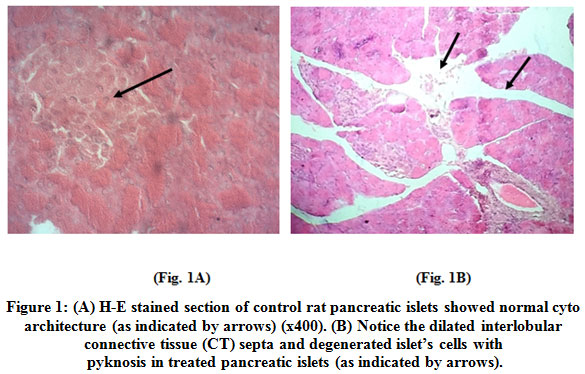 |
Figure 1: (A) H-E stained section of control rat pancreatic islets showed normal cyto architecture (as indicated by arrows) (x400). (B) Notice the dilated interlobular connective tissue (CT) septa and degenerated islet’s cells with pyknosis in treated pancreatic islet (as indicated by arrows). Click here to view figure |
.jpg) |
Figure 1: (C) Giemsa stained control rat pancreatic cells showing intact nuclei and membrane (as indicated by arrows) (x400). Click here to view figure |
.jpg) |
Figure 1: (D) Pyknotic pancreatic cells in treated rat group showed membrane rupture, necrosis and nuclear degeneration (x400). (E) Treated rat pancreatic cells displaying trypan blue (TB) positive response (as indicated by arrows) (x400). Click here to view figure |
Histopathological Findings and Analysis of Liver
Control liver tissues showed normal cytoarchitecture with visible central veins with radiating cords of hepatocytes (x100) (Fig.2A). The treated liver sections exhibited necrosis of hepatocytes, disruption of central canal, dilated sinusoidal spaces (Fig. 2B), central vein and vessel congestions (Fig. 2C) and periportal fatty infiltration (PFI) (Fig. 2D).
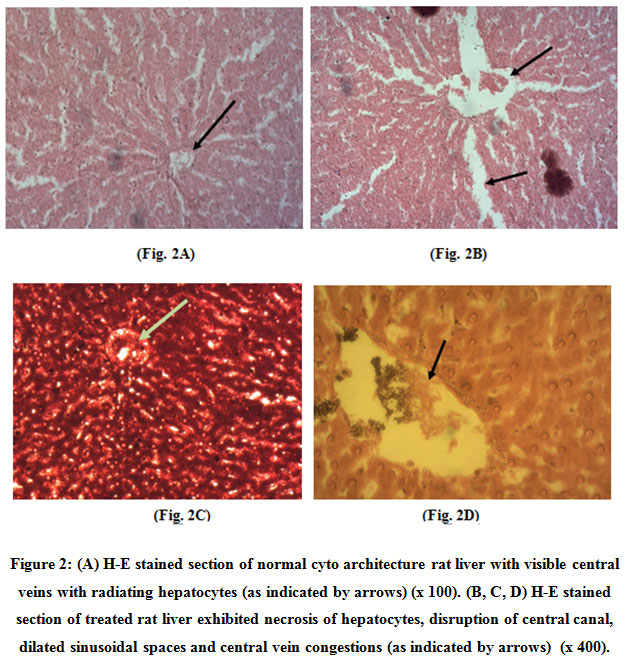 |
Figure 2: (A) H-E stained section of normal cyto architecture rat liver with visible central veins with radiating hepatocytes (as indicated by arrows) (x 100). (B, C, D) H-E stained section of treated rat liver exhibited necrosis of hepatocytes, disruption of central canal, dilated sinusoidal spaces and central vein congestions (as indicated by arrows) (x 400). Click here to view figure |
PAS Analysis of Liver
Liver section of treated group showed vacuolisation in the liver parenchyma. Treated liver showed lower (periodic acid/Schiff) PAS response (Fig. 3B).
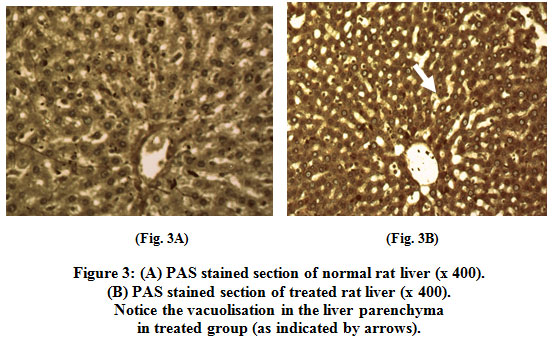 |
Figure 3: (A) PAS stained section of normal rat liver (x 400). (B) PAS stained section of treated rat liver (x 400). Notice the vacuolisation in the liver parenchyma in treated group (as indicated by arrows). Blood glucose level Click here to view figure |
The increased glucose level in treated rat didn’t return to control level even after 24 hr of glucose challenge (Fig. 4).
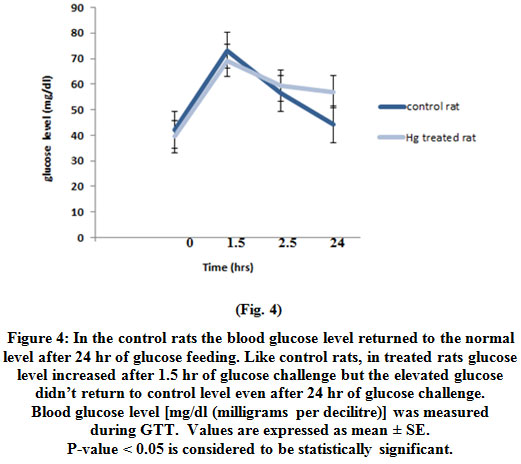 |
Figure 4: In the control rats the blood glucose level returned to the normal level after 24 hr of glucose feeding. Like control rats, in treated rats glucose level increased after 1.5 hr of glucose challenge but the elevated glucose didn’t return to control level even after 24 hr of glucose challenge. Blood glucose level [mg/dl (milligrams per decilitre)] was measured during GTT. Values are expressed as mean ± SE. P-value < 0.05 is considered to be statistically significant. Click here to view figure |
Liver and pancreas tissues both act as glucose sensor for diabetes mellitus (DM). In present study, histopathological examination of treated pancreas and liver showed the morphological alteration. Persistence of hyperglycemia was noticed in treated rat.
Chen et al., 2009 stated that islet cells were extremely sensitive to heavy metals due to high expression of metal transporters and low expression of antioxidants resulting in pancreatic islet β-cell dysfunction (Chen et al., 2009). Recent study evidenced that mercuric compounds (MeHgCl and HgCl2) caused pancreatic islet dysfunction by apoptosis [increasing apoptotic (p53, caspase-3) related gene expressions] and ROS generation in treated mice (Chen et al., 2012).
The detrimental effects of mercury act as negative regulators of insulin signaling and resistance by producing Reactive Oxygen Species (ROS) in cells (Durak et al., 2010; Bashan et al., 2009). Previous researches have also shown that group IIb metals (cadmium, mercury and zinc) modulates glucose transport in target cells (Barnes et al., 2003). Barnes and Kircher (2005) stated that pre-treatment with HgCl2 diminished glucose transport (Barnes and Kircher, 2005).
Guria, 2018 showed that the pancreatic sections and liver of the arsenic treated rat group exhibited marked morphological changes. Significant number of treated liver cells exhibited higher NBT (Nitroblue Tetrazolium) positive response (Guria, 2018). Alteration of glucose homeostasis was observed in arsenic treated rat (Guria, 2018). Guria et al., 2016 revealed that chromium (VI) had deleterious effect on the ultrastructure of pancreas as well as liver (Guria et al., 2016).
Conclusion
Recent study evidenced that mercuric compounds (MeHgCl and HgCl2) caused pancreatic islet dysfunction. This observation was consistent with earlier observations on genotoxic potential of mercury (II) chloride in liver, pancreas and other tissues.
The high expression of metal transporters in islet β-cells makes the islet cells extremely sensitive to the toxic effects of heavy metals, resulting in pancreatic islet β-cell dysfunction. Pancreatic islet β-cell degeneration and insulin resistance are the hallmark of Type2 diabetes mellitus, and thus heavy metals that reduce the function of β-cells are therefore highly relevant to T2D risk (Zheng et al., 2018; Edwards and Ackerman, 2016).
The result of present study corroborated the previous studies (Guria et al., 2016; Guria, 2018).
Therefore metal like mercury induced alteration of pancreas and liver may persuade the condition of diabetes mellitus. But further studies are needed to examine heavy metal exposures as risk factors for diabetes mellitus (DM).
Acknowledgement
Author states regards to the Head, Post Graduate Department of Zoology and Principal, Barasat Govt. College for providing infrastructure for conducting the work. The author would also like to thank Professor Madhusudan Das (Former Dean, Faculty Council for Post-Graduate Studies in Science, University of Calcutta), Department of Zoology, University of Calcutta, for his active cooperation.
References
- ATSDR (1999). Agency for toxic substances and disease registry. Toxicological profile for mercury. Atlanta, U.S. Department of Health and Human Services, Public Health Service, GA.
- Barnes DM and Kircher EA (2005). Effects of mercuric chloride on glucose transport in 3T3-L1 adipocytes. Toxicol. Vitro 19: 207-214.
CrossRef - Barnes DM, Hanlon PR and Kircher EA (2003). Effects of inorganic HgCl2 on adipogenesis. Tox. Sci 75: 368-377.
CrossRef - Bashan N, Kovsan J, Kachko I, Ovadia H and Rudich A (2009). Positive and negative regulation of insulin signaling by reactive oxygen and nitrogen species. Physiol. Rev 89: 27-71.
CrossRef - Chakrabarti S, Guria S, Samanta I and Das M (2007). Thyroid dysfunction modulates glucoregulatory mechanism in rat. Ind. J Exp. Biol 45 (6): 549-553.
- Chen KL, Liu SH, Su CC, Yen CC, Yang CY, Lee KI, Tang FC, Chen YW, Lu TH, Su YC and Huang CF (2012). Mercuric compounds induce pancreatic islets dysfunction and apoptosis in vivo. Int J Mol Sci 13: 12349-12366.
CrossRef - Chen YW, Huang CF, Yang CY, Yen CC, Tsai KS and Liu SH (2010). Inorganic mercury causes pancreatic β-cell death via the oxidative stress-induced apoptotic and necrotic pathways. Toxicol Appl Pharmacol 243: 323–331.
CrossRef - Chen YW, Yang CY, Huang CF, Hung DZ, Leung Y.M and Liu SH (2009). Heavy metals, islet function and diabetes development. Islets 1: 169–176.
CrossRef - Chen YW, Huang CF, Tsai KS, Yang RS, Yen CC, Yang CY, Lin-Shiau SY and Liu SH (2006). Methylmercury induces pancreatic β-cell apoptosis and dysfunction. Chem Res Toxicol 19: 1080–1085.
CrossRef - Durak D, Kalender S, Uzun FG, Demur F and Kalender Y (2010). Mercury chloride-induced oxidative stress in human erythrocytes and the effect of vitamins C and E in vitro. Afr. J. Biotech 9(4): 488-495.
- Edwards J and Ackerman C (2016). A Review of diabetes mellitus and exposure to the environmental toxicant cadmium with an emphasis on likely mechanisms of action. Curr Diabetes Rev 12(3): 252-258.
CrossRef - Guria S (2018). Arsenic induced hepatotoxicity and damage of pancreatic islets in albino rat: a possible role of diabetes mellitus. International Journal of Current Advanced Research 07(1): 9159-9163.
- Guria S (2017). Alteration of testicular cytomorphology in albino rats in alloxan induced diabetes: A histological study. J. Atoms and Molecules 7(5): 1133 – 1139.
- Guria S, Chakraborty B and Banerjee M (2016). Chromium (VI) induced histological changes of pancreatic islets and liver: a preliminary study of metal induced diabetes mellitus. The Experiment 35(1): 2171-2181.
- Guria S, Ghosh S and Das M (2014). Diabetogenic action of alloxan on liver histopathology. The Experiment 28(2): 1906-1912.
- Guria S, Chhetri S, Saha S, Chetri N, Singh G, Saha PB, Sarkar BS and Das M (2012). Study of cytomorphology of pancreatic islets and peritoneal macrophage in alloxan induced diabetic rat: a mechanistic insight. Animal Biology Journal 3(3): 101-110.
- Jomova K and Valko M (2011). Advances in metal-induced oxidative stress and human disease.
CrossRef - Toxicology 283: 65–87.
- Khan A R and Awan F R (2014). Metals in the pathogenesis of type 2 diabetes. J Diabetes Metab Disord 13:16. doi: 10.1186/2251-6581-13-16.
CrossRef - Marx J (2002). Unraveling the causes of diabetes. Science 296: 686-689.
CrossRef - Ratcliffe HE, Swanson GM and Fischer LJ (1996). Human exposure to mercury: A critical assessment of the evidence of adverse health effects. J Toxicol Environ Health 49: 221–270.
CrossRef - Shigenaga K (1976). Pancreatic islet injury induced by methyl mercuric chloride light and electron microscopic studies. Kumamoto Med. J 29: 67–81.
- Takeuchi T and Eto K (1997). Pathology and Pathogenesis of Minamata Disease. In Minamata
- Diseases Methyl Mercury Poisoning in Minamata and Niigata, Japan; Tsubaki, T., Irukayama, K.,
- Eds.; Kodansya: Tokyo, Japan 103–141.
- Uchino M, Tanaka Y, Ando Y, Yonehara T, Hara A, Mishima I, Okajima T and Ando M (1995). Neurologic features of chronic Minamata disease (organic mercury poisoning) and incidence of complications with aging. J Environ Sci Health B 30(5): 699–715.
CrossRef - Zheng T, Liu S, Bai Y, Cheng N, Buka S, Yang A, Shi K, Zhang X, Li Y, Xu S, Zhang B and Wise J (2018). Current understanding of the relationship between metal exposures and risk of type 2 diabetes. Curr Res Diabetes Obes J 7(2): CRDOJ.MS.ID.555710.
CrossRef






