Microbial Fuel Cells, Features and Developments
Mohsen Vaez1 , Shahareh Karami-Rad2 , Shohreh Tavakkoli2 and Hasan Diba3 *
DOI: http://dx.doi.org/10.12944/CWE.10.Special-Issue1.77
Current reliance on fossil fuels is unsustainable due to pollution and finite supplies. Microbial cell factories serve as promising alternatives renewable energy resources. Microorganisms generate electricity in their metabolism; act as catalysts for converting the chemical energy into electricity. In Microbial Fuel Cell (MFC), electrons provided by microorganisms flow through an electrical external circuit transport, create current and power. There are kind of MFCs such as Photosynthetic Alga Microbial Fuel Cells (PAMFCs), Microbial Desalination Cells (MDCs), and Sediment Microbial Fuel Cells (SMFCs). One of the main challenges with current state of MFCs biotechnology is its power output. MFCs with comparable power output can develop by terminal electron acceptors with a low redox potential and increase the cathode surface area. Anode and cathode performance are important factors limiting the power density of MFCs for practical application, but only a little development has been reported in the case of anode chamber.
Copy the following to cite this article:
Vaez M, Karami-Rad S,Tavakkoli S, Diba H. Microbial Fuel Cells, Features and Developments. Special Issue of Curr World Environ 2015;10(Special Issue May 2015). DOI:http://dx.doi.org/10.12944/CWE.10.Special-Issue1.77
Copy the following to cite this URL:
Vaez M, Karami-Rad S,Tavakkoli S, Diba H. Microbial Fuel Cells, Features and Developments. Special Issue of Curr World Environ 2015;10(Special Issue May 2015). Available from: http://www.cwejournal.org/?p=10894
Download article (pdf) Citation Manager Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2015-02-20 |
|---|---|
| Accepted: | 2015-03-30 |
Introduction
World needs to develop sustainable technologies to replace thousands of products have been generated from nonrenewable fossil fuels (Varman et al., 2013; Helder et al., 2012). New technologies in sustainable way are needed to facing challenges in the aspect of population explosion including global warming, freshwater scarcity, and soil deterioration (Wang, 2011). Microbial cell factories serve as promising alternatives for the production of diverse commodity chemicals and biofuels from renewable resources (Varman et al., 2013).
Microbial Fuel Cell
It has been known for almost one hundred years that microorganisms could generate electricity, but only in the past few years has this capability become more than a laboratory novelty (Barua and Deka, 2010). Microbial Fuel Cells (MFCs) are unique in promising sustainable energy biotechnology to utilize microorganisms as catalysts for converting the chemical energy of feedstock directly into electricity in their metabolism from what would otherwise be considered waste (Gruning et al., 2014; Aelterman et al., 2006; Barua and Daka, 2010; Mahendra and Mahavarkar, 2013), saline water (Cao et al., 2009), sediment (Jung et al., 2014; Franks and Nevin., 2010; Xie et al., 2010), or human excrement in space (Xie et al., 2010). MFCs present a complex microbial ecosystem similar to classical fuel cells, where the redox reaction is part of the microbial metabolism rather than mediated by an inorganic catalyst (Gruning et al., 2014).
Fig. 1 shows MFCs consist of two compartments separated by a proton exchange membrane (PEM). Biodegradable substrate electron donor oxidizes by anaerobic microorganisms’ respiration to obtain energy for their own growth in MFC anode chamber, pass through an external circuit in ion exchange membrane (IEM) to a separated cathode as terminal electron acceptor where an oxidant is reduced (Barua and Daka, 2010; Wang, 2011; Aelterman et al., 2006; Yadav et al., 2009; Mahesh et al., 2013)
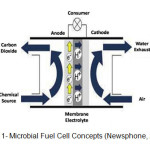 |
Figure 1: Microbial Fuel Cell Concepts (Newsphone, 2014). |
The transfer of electrons may occur by direct contact between membrane bound proteins and the electrode surface or by conduction through nanowires by microorganism that link cells to the electrode surface (Xie et al., 2010). While the electrons travel through the circuit, the corresponding protons migrate to the cathodic compartment through a proton exchange membrane (PEM) to maintain charge neutrality (Yadav et al., 2009).
Photosynthetic Alga Microbial Fuel Cell
The idea of developing biocathodes is to use the photosynthetic ability of microalgae for oxygen supply cathodic reaction (Yadav et al., 2009). Microalgae are a group of simple, plantlike, and old living organisms at the bottom of food chain in aquatic environments (Wan et al., 2013; Kumar and Sharma, 2014). Microalgae cover all unicellular and simple multicellular microorganisms including both prokaryotic cyanobacteria and eukaryotic green algae and diatoms (Li et al., 2008). Microalgae have the ability to reduce atmospheric CO2 into useful organic compounds by using solar energy (Varman et al., 2013). Photosynthetic Alga Microbial Fuel Cell (PAMFC) have emerged as a promising biotechnology for generating renewable energy since it relies on living organisms as inexpensive, self repairing and readily available catalysts to produce electricity from an abundant resource sunlight (Bombelli et al,, 2014). Power density of a PAMFC is determined by several aspects of the system such as solar radiation, photosynthetic efficiency of the microalgae, and efficiency of the MFC (Helder et al., 2012). PAMFCs capture and convert solar energy into electricity (Mahesh et al., 2013), using microalgae in the cathode compartment and a anaerobic microorganisms consortium in the anode (Gouveia et al., 2014). Entrapment of microalgae will reduce the area requirement for the production of more oxygen. It will lead to development of compact cathode of MFC and will reduce the higher internal resistance problem, which is one of the MFC scaling up bottlenecks (Yadav et al., 2009).
Microbial Desalination Cell
Halophiles are salt loving microorganisms optimally growing at high concentrations of salt with the capacity to balance the osmotic pressure and resist the denaturing effects of salts (Diba et al., 2014). The Microbial Desalination Cell (MDC) is a promising biotechnology to desalinate water without electrical energy input (Shehab et al., 2013). Based on MFC’s structure, it could have fulfilled desalination function by addition of a middle chamber and anion exchange membrane (AEM) (Wang, 2011). In fig. 2 concept of a PAMFC modified to MDC is shown. MDCs consist of three compartments, the anode, the cathode, and a salt compartment, which is between the anode and the cathode (Brastad and He, 2006).
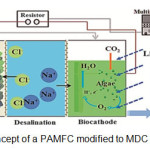 |
Figure 2: Concept of a PAMFC modified to MDC (Blogs, 2013). |
MFCs with AEMs often show better performance than those with cation exchange membrane (CEMs). The primary species transferred across the membrane with an AEM, are Clˉ, HCO3ˉ, and HPO42- along with OHˉ ions. While ionic imbalances are detrimental to the general operation of MFCs, the movement of ions across membranes during current generation provides a method for altering water chemistry in a manner that can be useful for achieving water desalination (Cao et al., 2009).
Sediment Microbial Fuel Cell
A sediment Microbial Fuel Cell (SMFC) is a device that produces electricity biologically from sediments in aquatics, can be a feasible solution for sustainable power generation in remote places, too (Jung et al., 2014; Franks and Nevin, 2010).
Discussion
Current reliance on fossil fuels is unsustainable due to pollution and finite supplies (Franks and Nevin, 2010). With increasing urbanization, the challenge for both clean water and alternative sources of renewable energy are urgently required to sustainability (Limson et al., 2013).
With increasing concerns for alternative energy sources, the investigation of MFCs has received considerable attention in recent years. Plenty of electrons derived from organic or nonorganic energy sources, stored in waste biomass of natural, agricultural, municipal and industrial waste (El-Naggara et al., 2010), used by anaerobic or facultative anaerobic bacteria in anodic microbial catalysts in MFC design (Limson et al., 2013). It is estimated that in the next 20 years the average per capita supply of clean water will decrease by one-third. Desalination is one option for producing potable water from brackish water and seawater in many parts of the world, but most water desalination technologies need energy and intensive (Cao et al., 2009). Halophiles in DMCs desalinate water without need of electrical energy input (Shehab er al., 2013). One of the main challenges with current state of MFCs biotechnology is its power output. Maximum power outputs of the PAMFC have been achieved in short term tests like polarization curves and were not sustained for longer periods of time. A long distance from anode to cathode leads to transport losses in the anode. In some researches the design of anode chamber has tubular shape with a membrane at the bottom of the tube, where cathode is situated underneath the anode (Helder et al., 2012).
In MFCs, microorganisms consume a substrate in anaerobic conditions, produce carbon dioxide, protons and electrons in anode chamber (Pranab and Deka, 2010). Anode performance is an important factor limiting the power density of MFCs for practical application but only a little development has been reported. To improve the interaction between the anode surface and microbial biofilm, researchers have employed various commercially available carbon based porous anodes including carbon cloth, carbon paper, carbon foam, and reticulated vitrified carbon (Xie et al., 2010). The performance of MFC may be enhanced through several important process parameters including cell metabolism, microbial electron transfer, proton exchange membrane transfer, external and internal resistances, and cathode oxidation (Rahimnejad et al., 2014). The performance of the cathode as anode, is an important limitation due to usage of sustainable catholyte (Mahesh et al., 2013), Generally, oxygen is used as the final electron acceptor; accelerate the water formation process in the cathode chamber (Rahimnejad et al., 2014). The distances which the charge carriers have to migrate within the devices can be shortened dramatically, reducing resistive losses in the electrolyte (Bombelli et al., 2014).
PAMFC is a microalgae biotechnology utilize in cathode chamber that could produce sustainable bioelectricity by producing oxygen in their metabolism (Helder et al. 2012). The demonstrated possibility of producing added value microalgae biomass in microbial fuel cell cathodes will increase the economic feasibility of bioelectrochemical systems, allowing the development of energy efficient systems for desalination or wastewater treatment and carbon fixation (Gouveia et al., 2014). The use of algae as a feedstock in MFCs has not been widely reported in the literature (Limson et al., 2013).
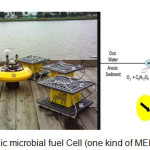 |
Figure 3: A benthic microbial fuel Cell (one kind of MEDIC) (NRL, 2013). |
Water desalination can be accomplished without electrical energy input or high water pressure by using a source of organic matter as the fuel to desalinate water (Cao et al., 2009). In MDCs, during the desalination process, the anodic bacteria are still faced with high salinity shock. Changes of anodic microbes in high TDS[1] MFCs were not clear. The microbial community may be changed by salinity to halotolerants due to succession. Addition of salts increases the conductivity and reduces the internal resistance, thus is beneficial to electricity generation and removing TDS (Zhang et al., 2011). MDC modified by adding a desalination chamber between the anode and cathode. Exoelectrogen microorganisms catalyze the conversion of electron donors to bioelectricity. The internal resistance in MDCs increase significantly with decreasing water salinity, needs to optimize this technology to achieve higher desalination rates and power densities (Shehab et al., 2013). In a modified MDC by Cao et al. (2009), two membranes placed between the anode and cathode, creating a middle chamber for water desalination between the membranes. An anion exchange membrane was placed adjacent to the anode, and a cation exchange membrane was positioned next to the cathode. When current was produced by bacteria on the anode, ionic species in the middle chamber were transferred into the two electrode chambers, desalinating the water in the middle chamber. Through adjustment of the flow rates into electrode chambers and desalination chamber, it should be possible to limit pH changes to desired levels. The addition of proportional buffer and the use of anode to cathode recirculation are two possible approaches for addressing this problem (Cao et al., 2009). In order to increase the MFC power density, internal resistance should be decreased (Helder et al., 2012).
MEDIC reactors showed better performance in desalination and energy recovery than MDC reactors. These differences were due to the presence of the ion exchange resins (IERs), which could act as a bridge to enhance ion transfer between the solution and ion exchange membranes. In MEDIC reactors, bacteria in the anode chamber oxidized the organic matter faster than the MDC reactors and have higher productivity of salt removal than MDCs (Shehab et al., 2013). In fig. 3 a benthic microbial fuel cell has shown (it’s a kind of MEDICs).
Between an anode surface and microbial biofilms to facilitate extracellular electron transfer, besides high conductivity, chemical stability, biocompatibility, resistance to decomposition, and catalytic activity, optimal anodes preferably require porous structure to allow internal colonization and strong interaction including affinitive mechanical contact and higher electrical conductivity (Xie et al., 2010). MFCs with comparable power output can develop by terminal electron acceptors with a low redox potential (Gruning et al., 2014) and increase the cathode surface area (Banik et al., 2012). MFC with O2 or air as the cathode electron acceptor needs expensive platinum as catalyst to accelerate the O2 reduction (Mahesh et al., 2013). The improvement of the performance of the oxygen reduction at lowest possible costs (Yadav et al., 2009) or novel anaerobic microorganism as cathode catalyst is one of the most important issues for research and development (Mahesh et al., 2013). A number of mediators have been suggested for use in microbial fuel cells, including natural red, methylene blue, thionine or resorfuin (Pranab and Daka, 2010).
To further improve MFC technology an understanding of the limitations and microbiology of these systems is required. Some researchers are uncovering that the greatest value of MFC technology may not be the production of electricity but the ability of electrode associated microbes to degrade wastes and toxic chemicals (Franks and Nevin, 2010).
Alternative energy production strategies are gaining importance for combating future energy crisis. MFCs could be an effective means to enhance bioelectricity production. This review was unable to examine the entire field of MFC research in detail but hopes to highlight some important points.
The discovery that microbial metabolism could provide energy in the form of an electrical current has lead to an increasing interest and a dramatic raise in the number of publications in the field of MFC research. One of the most active areas of MFC research is the production of power from wastewaters. Studies are demonstrating that any compound degradable by bacteria can be converted into electricity (Franks and Nevin, 2010). Generated electrons provided from chemical bonds with the aid of active microorganisms, flow through an electrical external circuit transport to anode, produced protons are moved through a proton exchange membrane toward the cathode compartment. The flow of electrons create current and power (Rahimnejad et al., 2014).
In PAMFC, microalgae use to supply oxygen by photosynthesis in the cathode chamber (Gouveia et al., 2014). It should be noted that in this system microorganisms utilized both in the cathode and anode compartments, describe a green system.
MFCs still face problems of scaling up from laboratory experiments. Various metals have typically been used to catalyze the cathodic reaction but oxygen reduction cathode is currently an important limiting factor in a MFC (Franks and Nevin, 2010). Improving the anode design is important for enhancing the MFC performance but only a little development has been reported. The performance of MFCs is affected by many factors, including the cathodic reaction, substrate, buffer system, and operating temperature (Xie et al., 2010). Further studies are necessary to understand the effect of different operational parameters and optimize the electricity production from the MFC. With continuous improvements in microbial fuel cell, it may be possible to increase power generation and reduce production and operating cost of MFCs. Thus, the combination of wastewater treatment along with electricity production may help in compensating the cost of wastewater treatment, making it sustainable (Ghangrekar and Shehab, 2006).
References
- Aelterman, P; Rabaey, K; Clauwaert, P. and Verstraete, W. Microbial fuel cells for wastewater treatment. Water Science & Technology, 54(8), 9-15 (2006).
- Banik, A; Jana, N. K; Maiti, M. R; Ghosh, T. K. and Greener, B. Development of Microbial Fuel Cells and Electrode Designs with Waste Water Anaerobes. Greener Journal of Biological Sciences, 2(2), 13-19 (2012).
- P. K. and Deka, D. Electricity Generation from Biowaste Based Microbial Fuel Cells. International Journal of Energy, Information and Communications, 1(1) (2010).
- Bio-cathodes: powering towards clean water, energy and biomass production. Retrieved 13 November 2014 from: http://blogs.rsc.org (2013).
- P; Uller, M. B; Herling, T. W; Chrostopher, J. H. and Knowles, Y. P. J. A High Power-Density Mediator-Free Microuidic Biophotovoltaic Device for Cyanobacterial Cells. Advanced Energy Materials (2014).
- Brastad K. S. and He, Z. Water softening using microbial desalination cell technology. Desalination, 309, 32-37 (2013).
- Cao, X; Huang, X; Liang, P; Xiao, K; Zhou, Y. and et al. A New Method for Water Desalination Using Microbial Desalination. A American Chemical Society, 43(18), 7148–7152 (2009).
- Diba, H; Hemmat, J. and Karimi, S. Biofouling of Reverse Osmosis (RO) Membranes: Present and Future. 2nd International Training Workshop, Conference and Exhibition on Desalination, Iranian Research Organization for Science and Technology, Tehran (2014).
- El-Naggara, M. Y; Wangerb, G; Leungc, K. M; Yuzvinskya, T. D; Southame, G. and et al. Electrical transport along bacterial nanowires from Shewanella oneidensis MR-1. PNAS, 107(42), 18127–18131 (2010).
- Franks, A. E. and Nevin, K. P. Microbial Fuel Cells, A Current Review. Energies, 3, 899-919 (2010).
- Ghangrekar, M. M. and Shehab, N. A. Wastewater Treatment in Microbial Fuel Cell and Electricity Generation: A Sustainable Approach. 12th international sustainable development research (2006).
- Gouveia, L; Neves, C; Sebastiano, D; Nobre, B. P. and Matos, C. T. Effect of light on the production of bioelectricity and added-value microalgae biomass in a Photosynthetic Alga Microbial Fuel Cell. Bioresource Technology, 154, 171–177 (2014).
- Gruning, A.; Beecroft, N. J. and Avignone-Rossa, C. Metabolic composition of anode community predicts electrical power in microbial fuel cells. Retrieved 1 January 2015 from: https://archive.org/details/biorxiv-10.1101-002337 (2014).
- Helder, M; Strick, D. P; Hamelers, H. V. and Buisman, C. The flat-plate plant-microbial fuel cell: the effect of a new design on internal resistances. Biotechnology for Biofuels, 5 (2012).
- Jung, S. P; Yoon, M; Oh, L. S., S; Kang, H. and Yang, J. Power Generation and Anode Bacterial Community Compositions of Sediment Fuel Cells Differing in Anode Materials and Carbon Sources. International Journal of Electrochemical Science, 9, 315-326 (2014).
- Kumar M. and Sharma, M. V. Potential assessment of microalgal oils for biodiesel production: A review. Journal of Materials and Environmental Science, 5(3), 757-766 (2014).
- Li, Y; Horsman, M. Wu, N; Lan, C. Q. and Dubios-Calero, N. Biocatalysts and Bioreactors Design. Biotechnology Advances, 24, 815- 820 (2008).
- Limson, J; Mtambanengwe, K; Mshoperi, E; Fogel, R. and Laubscher, R. The beneficiation of algal biomass from a low-cost sewage treatment system as a feedstock in microbial fuel cells, ECS Meeting (2013).
- Mahendra, B. C. and Mahavarkar, S. Treatment of Wastewater and Electricity Generation using Fuel cell technology. Retrieved 1 January 2015 from: doi.org/10.15623/ijret.2013.0213050 (2014)
- Mahesh, M; Desalegn, T. and Alemayehu, M. Evaluation of Photosynthetic Microbial Fuel Cell for Bioelectricity Production. Indian Journal of Energy, 2(4) (2013).
- How do fuel cells work. Retrieved 16 desember 2014 from: http://newsphone.ga (2014).
- Continuous Sustainable Power Supply: Benthic Microbial Fuel Cell. Retrieved 16 Desember 2014 from: http://www.nrl.navy.mil (2013).
- Pranab, K. and Deka, D. Electricity Generation from Biowaste Based Microbial Fuel Cells. International Journal of Energy, Information and Communications, 1(1) (2010).
- Rahimnejad, M; Bakeri, G; Najafpour, G; Ghasemi, M. and Oh, S. Review on the effect of proton exchange membranes in microbial fuel cells. Biofuel Research Journal, 1, 7-15 (2014).
- Shehab, N. A; Logan, B. E; Amy, G. L. and Saikaly , P.E. Microbial Electrodeionization Cell Stack for Sustainable Desalination, Wastewater Treatment and Energy Recovery. Retrieved 6 January 2015 from: http://www.researchgate.net/publication/257943930_Microbial_Electrodeionization_Cell_Stack_for_Sustainable_Desalination_Wastewater_Treatment_and_Energy_Recovery (2013).
- Varman, A. M; Yu, Y. and Tang, Y. J. Photoautotrophic production of D-lactic acid in an engineered Cyanobacterium. Microbial Cell Factories, 12(117) (2013).
- Wan, C; Bai, F. W. and Zhao, Q. X. Effects of nitrogen concentration and media replacement on cell growth and lipid production of oleaginous marine microalga Nannochloropsis oceanica DUT01. Biochemical Engineering Journal, 78, 32-38 (2013).
- Wang, H. Ammonium Removal and Electricity Generation by Using Microbial Desalination Cells (Royal Institute of Technology (KTH), 2011).
- Xie, X; Hu, L; Pasta, M; Wells, G. F; Kong, D. and et al. Three-Dimentional Carbon Nannotube- Textile Anode for High- performance Microbial Fuel Cells. Nanno Lifters, 11(1), 291–296 (2010).
- Yadav, A. K; Panda, P; Rout, P; Behara, S; Patra, A. K. and et al. Entrapment of algae for wastewater treatment and bioelectricity generation in Microbial fuel cell. 17th International Conference on Bioencapsulation. Groningen, Netherlands (2009).
- Zhang, L; Ding, L., Li, C., Xu, K. and Ren, H. Effects of electrolyte total dissolved solids (TDS) on performance and anodic microbes of microbial fuel cells. African Journal of Biotechnology, 10(74), 16909-16914 (2011).







