Oryzalin Treatment Modified Plant Morphology of Impatiens balsamina L.
M. Ria Defiani1 * , D. N. Suprapta2 , I. M. Sudana3 and N.Putu Ristiati4
1
Udayana University,
Denpasar Bali,
Indonesia
2
Laboratory of Bio Pesticide,
Faculty of Agriculture,
Udayana University,
Denpasar Bali,
Indonesia
3
Faculty of Agriculture,
Udayana University,
Denpasar Bali,
Indonesia
4
Department of Biology Education,
Faculty of Natural and Basic Sciences,
Ganesha Education University,
Singaraja Bali,
Indonesia
DOI: http://dx.doi.org/10.12944/CWE.8.1.03
Copy the following to cite this article:
Defiani M. R, Suprata D. N, Sudana I. M, Ristiati N. P. Oryzalin Treatment Modified Plant Morphology of Impatiens balsamina L. Curr World Environ 2013;8(1) DOI:http://dx.doi.org/10.12944/CWE.8.1.03
Copy the following to cite this URL:
Defiani M. R, Suprata D. N, Sudana I. M, Ristiati N. P. Oryzalin Treatment Modified Plant Morphology of Impatiens balsamina L. Curr World Environ 2013;8(1). Available from: http://www.cwejournal.org/?p=3301
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2013-02-25 |
|---|---|
| Accepted: | 2013-03-12 |
Introduction
Impatiens balsamina L. (garden balsam) is a flowering plant that belonged to Balsaminaceae family. The plant has many flower colour like red, purple, pink and white. The flowers can be used for gardening and potted plants, nail polished and natural colouring agent,1 antibiotic activity against pathogenic bacteria and fungi,2 offerings in Balinese ceremony.3 Seed extract of garden balsam contained antimicrobial activity against the growth of E.coli and Bacillus anthrasis and antifungal against Aspergillus niger and Fusarium sp.4 Seeds also used for expectorant and treatment for cancer.5 This plant is relatively tall with plant height6 can reach up to 65 cm or higher that depend on type and fertility of soil.In the field, tall plants are susceptible to be lodged by strong wind and heavy rain.3 Previously, colchicine is widely applied for plant modification such as Rhododendron to produce compact plants,7 Impatiens balsamina L. to obtain tetraploid plants,8 Phlox subulata,9 ornamental ginger (Hedychium muluense),10 basil (Ocimum basilicum L.)11 and yellow passion fruit,12 Portulaca grandiflora13 to increase flower size. In fact, colchicine in a certain concentration had negative effect to the plant cell,14 environment15 and people who are exposed to this chemical.16 In contrast, oryzalin is easier to be degradabled by light and had similar effect to colchicineas antimitotic agent in order to inhibit mitosis.12 Oryzalin is a herbicide that can be used for inhibit root growth.17 As herbicide, oryzalin was applied preemergence for control seedlings of grasses and annual broadleaves plants.17
As mutagenic agent, oryzalin was used in some plants such as rose18 and Mecardonia tenella a native plant from South America19 to produce shorter plant with bigger flower, Rhododendron to obtain thicker leaf and vigorous plant,7 Hibiscus acetosella to have compact plant form,20 banana cultivar to increase microshoot production.21 Oryzalin can replace colchicine application as antimitotic agent, that bind tubulin dimmer along division of cell and alter microtubules formation and spindle fibers.22 Based on those previous study, oryzalin was applied to treat seedlings of Impatiens balsamina to modify plant form. Preliminary study showed that the growth of radicles of garden balsam seedlings was altered when treated with oryzalin because roots elongation was very poor.3 After 7 days of germination, control and treatment with 0.01% oryzalin showed the growth of roots and hypocotyl. In contrast, treatment with oryzalin higher than 0.01 % only showed the growth of hypocotyl, while the roots was very slow and stunted. Effects of oryzalin in the field varied between plant species and oryzalin concentrations. In order to have shorter and compact plants, garden balsam seedlings were treated with oryzalin as a mutagenic agent with various time of soaking. Vigorous plant has advantage as border plant in a landscape or potted plants. In addition, lowered plants can enhance plant survival during heavy rain.
Materials and Methods
Oryzalin treatment
Seeds of garden balsam with red flower were pretreated by soaking in distilled water for 12 h and germinated on filter paper in Petri Dishes. Germinated seeds (4 days old) were treated with oryzalin in different concentrations (0, 0.01, 0.02, 0.03)% for (0,12, 24, 36 and 48) h. Treated seedlings were rinsed in water and planted in polybags and grown in the field. The randomized block design was applied to allocate the treatment. There are 20 of combination treatments and each treatment was repeated three times. Thus, there are 60 experimental units and each of experimental unit consisting of 5 plants. The plants were maintained by watering every day and applying fertilizer (NPK 15:15:15) at 4 weeks and 8 weeks intervals after planted.
Plant Morphology
Morphology measurement includes plant height (from the stem base to the shoot tip), number of branch, weight of flower.
Flowcytometry analysis (FCM)
FCM analysis was sampled from M3 generation. Each treated plant that showed altered growth with bigger flower size was analysed to check its ploidy level. Sample of leaf tissue (0.5 cm2) was put in 55 mm plastic Petri Dishes (Partec code 04-2005) and was added with 500 µL extraction buffer (Partec Kit). Material was chooped using a sharp razor blade for 30 to 60 seconds, then incubated for 90 seconds before filtered through a Partec 50 µm Cell Trics disposable filter (code 04-0042-2317). Staining solution (PI) and RNAse of 2 mL each were added to the test tube, then incubated and protected from light at least 30 min. Sample was then analysed with flow cytometer in the red channel (Partec GmbH Flow Cytometry, Germany).
Statistical analysis
All data were subjected to the analysis of variance (ANOVA) followed by Duncan’s Multiple Range Test (DMRT) at 5% level (CoStat Co.).
Result and Discussion
Plant heightTwelve weeks after planted, plant height was measured to know the effect of oryzalin and time of incubation for plant performance. M1 generation showed significance of different for interaction of the treatment between oryzalin concentration and time of incubation on plant height (Table 1). Concentration of oryzalin 0.01% for 48 h and oryzalin 0.03% for 48 h reduced plant height significantly by 53.6 % and 53.9 %, respectively when compared to control.
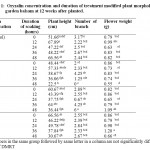 |
Table 1: Oryzalin concentration and duration of treatment modified plant morphology of garden balsam at 12 weeks after planted. Click here to View table |
Plant height of Hibiscus acetosella treated with colchicine and oryzalin were reduced and internode were shorter in octoploid plants when compared with diploid.20 Reduction of plant size had reported for some polyploid plant. In arrowroot (Maranta arundinacea) plant, oryzalin treatment at high concentration (≥30 µM) inhibited plant growth. In contrast, at concentration of 10 µM enhanced growth of plant.23 Mecardonia tenella treated with colchicine 0.01% for 48 h incubation produced tetraploid plant in the field and showed shorter and more compact plant than diploid.19
Number of Branch
Plant response to oryzalin treatment was varied in number of branch. The plants treated with 0.01% oryzalin for 48 h incubation did not produce any branch, eventhough higher concentration of oryzalin 0.02% for 48 h incubation produced 6 branches (Table 1), and significantly different to control. Oryzalin treatment altered the growth of vegetative plants. Treated plant with 0.01 % for 48 h showed the shortest plant without any branch, the lowest weight and diameters of flower. In addition, in M1 generation, the treatment did not produce polyploid plant, but plant morphology showed a dwarf plant that suitable for potted plant or border plant in landscape garden. Inhibition of vegetative growth due to limited root growth after treated with oryzalin. Radicle was very slow to develop longer roots and even fail to produce root hairs.
Seedlings stage is very critical. Limitation on root growth can alter further growth of shoots because the absorption of nutrients were very low.
Flower weight
Weight of flower was not statistically different between all treatments including control in M1 generation (Table 1). Oryzalin 0.02% for 48 h incubation and 0.03% for 36 h incubation tended to produced more weight of flower (32.9 % and 48.8%, respectively).
The flower weight did not influenced by flower diameter. Increased in flower weight did not followed by enhanced of flower diameter (data not shown). Visually, flower with higher in weight obtained more petals eventhough in small size of petals. In Rose, tetraploid plants showed double the number of petals flower.18 Tetraploid plants due to colchicine treatment of Portulaca grandiflora had a large number of petals than diploid plants13 (Mishiba and Mii, 2000). Mecardonia tenella treated with colchicine 0.01% for 48 h then cultured in vitro, obtained bigger flowers compared to control plants.19 In the field, compact shaped were shown by selected tetraploid plants of M. Tenella.
Ploidy Analysis by FCM
Ploidy level of garden balsam was analysed by flowcytometry for further generation (M3). Concentration oryzalin 0.02% for 12 and 24 h incubation and oryzalin 0.03 % for 12 h, 36 h, 48 h showed mixoploid plants (2x+4x) (Fig. 1). Based on FCM analysis, oryzalin treatment to seedlings of garden balsam was unsuccesfull in inducing tetraploid plants on M3 generation, however mixoploid plants were obtained in the present study. Mixoploid plant (2x+4x) had shorter plant morphology and higher number of petal flower than diploid plants (Fig. 2).
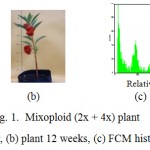 |
Figure 1: Mixoploid (2x + 4x) plant Click here to View figure |
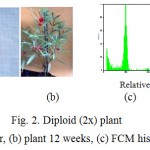 |
Figure 1: Diploid (2x) plant Click here to View figure |
Acknowledgements
The authors thanks to Mr Fajarudin Ahmad from Indonesian Institute of Science for his assisstant in flowcytometry analysis.
Refrences
- Polunin O. and Stainton A., Flowers of the Himalayas. Oxford Universtiy Press (1984).
- Chopra R. N., Nayar S.L. and Chopra I.J., Glossary of Indian Medicinal Plants (Including the Supplement). Council of Scientific and Industrial Research, New Delhi (1986).
- Defiani M.R., Procceeding International Conference on Biological Science. Gajah Mada University, Indonesia, (2011).
- Jain B., Curr. World Enviro. J., 6, 299 (2011).
- Duke J.A. and Ayensu E.S., Medicinal Plants of China. Reference Publications, Inc. (1985).
- Anurita D. and Girjesh K. , Caryologia, 60, 199 (2007).
- Vainola A., Euphytica, 112, 239 (2000), http://dx.doi.org/10.1023/A:1003994800440
- Wiendra N.M.S., Undergraduate Thesis, Universitas Udayana, Denpasar Bali Indonesia (2008).
- Zhang Z., Dai and Xiao M. Euphytica, 159, 59 (2008), http://dx.doi.org/10.1007/s10681-007-9457-8
- Sakhanokho H.F., Rajasekaran K., Kelley R.Y. and Islam-Faridi N., HortSci., 44, 1809 (2009).
- Omidbaiqi R., Mirzaee M., Hassani M.E.and Moghadam M.S., Intl. J. of Plant Prod., 4, 87 (2010).
- Rego M.M., Rego E.R., Bruckner C.H., Finger F.L. and Otoni W.C., Plant Cell Tiss. Organ Cult. 107, 451(2011), http://dx.doi.org/10.1007/s11240-011-9995-6
- Mishiba K. and Mii M., Plant Sci.,156, 213 (2000), http://dx.doi.org/10.1016/S0168-9452(00)00257-0
- Jaap M., Van Tuyl B., Meijer and van Dien M.P., Acta Hort., 325, 625 (1992).
- Hanumante M.M., Vaidya D.P. and Nagabhushanam R., Bull. Environ, Contam and Toxic, 24, 37 (1980), http://dx.doi.org/10.1007/BF01608072
- Finkelstein Y.S.E.Aks., Hutson J.R., Juurlink D.N., Nguyen P., Dubnov-Raz U., Pollak G. Koren and Bentur T., Clin. Toxicol, 48, 407 (2010), http://dx.doi.org/10.3109/15563650.2010.495348
- Ross M.A. and Childs D.J., Herbicide Mode of Action Summary. Department of Botany and Plant Pathology, Purdue University (1994).
- Kermani M.J., Sarasan V., Roberts A.V., Yokoya K., Wentworth J. and Sieber V.K., Theo. and Appl. Gen., 107,1195 (2003), http://dx.doi.org/10.1007/s00122-003-1374-1
- Escandon A.S., Alderete L.M. and Hagiwara J.C., Sci. Hort., 115, 56 (2007), http://dx.doi.org/10.1016/j.scienta.2007.07.006
- Contreras R.N., Ruter J.M. and Hanna W.W., J.Amer.Soc. Hort. Sci., 134, 553 (2009).
- Ganga M.and Chezhiyan N., J. Hort. Sci. Biotech., 77, 572 (2009).
- Petersen K.K., Hagberg P. and Kristiansen K., Plant Cell, Tiss.Organ Cult., 73, 137 (2003), http://dx.doi.org/10.1023/A:1022854303371
- Sukamto L.A., Ahmad F. and Wawo A.H., Bul. Littro, 21, 93 (2010).






