Removal of heavy metals from waste water using different biosorbents
S. Murugavelh1 * and D.Vinothkumar 2
DOI: http://dx.doi.org/10.12944/CWE.5.2.12
The discharge of waste water containing heavy metals like Cr, Cd, Cu from tanneries, electroplating units, metal processing industry etc is the major cause for metal pollution which in turn causes adverse effects in the environment. The conventional methods available for removal of heavy metal are costly and also produce some toxic sludge. This has lead to the invention of new technology like biological removal of heavy metal. In this paper use of various biosorbents like plants, microbes and their derived products were reviewed with context to metal removal. Various modes of operation and reactor types, adsorption kinetics with respect to growth condition and metal sorption were also given importance.
Copy the following to cite this article:
Murugavelh S, Vinothkumar D. Removal of heavy metals from waste water using different biosorbents. Curr World Environ 2010;5(2):299-304 DOI:http://dx.doi.org/10.12944/CWE.5.2.12
Copy the following to cite this URL:
Murugavelh S, Vinothkumar D. Removal of heavy metals from waste water using different biosorbents. Curr World Environ 2010;5(2):299-304. Available from: http://www.cwejournal.org/?p=1201
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2010-11-19 |
|---|---|
| Accepted: | 2010-12-16 |
Introduction
Industrial revolution has lead to the increased use of heavy metal like Cr, Cu, Cd, Pd, Zn etc. Heavy metals such as Cr, Cu, Cd, Pd, Zn are essential for the plants and microbes as they form part of many enzymes and proteins.1 The effluents from metal processing industries and tanneries are released into the environment without proper treatment or untreated. These metals accumulate on the soil and are persistent and pose a severe threat to the environment. Elevated level of both essential and non essential metals has lead to toxicity to living organisms. To protect themselves from metal poisoning plants and microbes have developed a mechanism by which the metal entering their cells are inactivated or transformed to nontoxic forms. The conventional methods available for metal removal are chemical treatment like precipitation, reverse osmosis and thermal treatment. Chemical treatment of metal results in production of sludge which is much more dangerous than metal solution. Other methods like reverse osmosis are efficient but costly, these limitations has resulted in innovation of new techniques like biosorption and bioremediation. Literature review reveals that various plants like aquatic plants,1,3 neem bark, husk, microbial sources like algae, fungi, yeast,4,5 bacteria can be used as efficiently used as biosorbent for heavy metal removal. The biosorbents have advantage over conventional methods in low cost, high efficiency, regeneration.Biosorption is the recent trend for removal of recalcitrant elements. It utilizes the ability of biological materials to accumulate heavy metals from wastewater streams by either metabolically mediated or physic-chemical pathway of uptake.1 Metabolically inactive biomass due to their unique chemical composition sequesters metal ions and metal complexes from solution. Metal sorption by inactive biomass is advantageous that it does not need the maintenance of specific growth conditions.2 Bioremediation utilizing plants or microbes for removal of heavy metal is a suitable alternative for conventional methods, but require the maintenance of special growth conditions. Bioremediation is aesthetically pleasing and makes environment green as the entire process is solar energy driven making it cost effective and environment friendly.
Immobilization is the process by which the whole cell or the metabolites like enzymes are attached on to an inert, insoluble material like calcium alginate, polyurethane foam, etc. immobilization gives the advantage that the microbes can with stand increased stress like change in pH , temperature. Immobilized microbes were tested in their efficiency for metal removal. Various microbes on different support matrix have been reviewed.
Mechanism of metal uptake by biosorbents
It is necessary to know the mechanism by which biological materials accumulate metals. Microbial cells contain a large number of metal binding sites called ligands. Biomass cell wall contains polysaccharides proteins and lipids which offer functional groups to bind metal ions.4 The mechanism by which biological materials remove metals is not completely understood. The performance of the biosorbent depends on the ionic state of the biomass. The uptake of metals by biomass is a two step process, the cells of biomass contains proteins and polysaccharides which offer a lot of binding site for heavy metals. The first step is the stoichiometric interaction between the cell components and the metal ions. The second step is the accumulation of heavy metal on the binding sites.15
Biosorption is a complex process comprosing of ion exchange, chelation and adsorption by physical forces, entrapment in capillaries. Acetamide groups of chitin, amino and phosphate groups of nucleic acid, amide and sulfhydryl groups of proteins are well known examples of chemical groups that attract metals in biomass. The steric and conformational effects also have a major role in binding capacity of biomass.
Plants as biosorbents
Removal of heavy metals from aqueous solutions using plants and plant derived biomass is a viable option when cost of removal is a major criterion. Large numbers of works have been performed with various plant based biomass. Eicchornia crassipes, sawdust, bael fruit, wheat straw have been reviewed as biosorbents.
Reed
Reed contains lignin and cellulose as their cellular component which has the ability to adsorb heavy metal ions from metal solutions. Bounheng et al., 2006 studied the use of reeds as biosorbent for removal of heavymetals. Reeds are cut into small pieces and washed with distilled water and dried to 60°C and ground to homogenous powder. Pretreatment of reed is done treatment with acid and alkali. 0.05g of reed sorbent so prepared was treated against metal solution at a pH range of 2-3 and for 3h in a shake flask. Plasma absorption spectroscopic studies showed 64% removal of heavy metals.6
Saw dust
Saw dust from work shops were investigated for removal of Cr. The pretreatment step involves the washing of saw dust with distilled water and dried at room temperature for 8h. Batch study was performed with addition of various concentration of saw dust to flasks containing Cr solutions (1000mgL-1) at 25°C. The concentration of the chromium is determined by spectrometric method. Tarun et al., 2009 reported 42.52 mg g-1 adsorption of chromium for a pH range of 1. Various adsorption isotherms were also studied. Langmuir isotherm was found to best fit the results.7
Sunflower stem waste
Sunflower stem was sundried and ground in ball mill and washed with distilled water, oven dried at 60°C and sieved to 300 µm. Pretreatment was done with water and formaldehyde. Batch adsorption study was done with treatment of (1000mgL-1) of chromium solution in a flask with a solution pH of 2 for (0.2 mg of biosorbent/ 50ml of chromium solution. The solution was centrifuged at 400 rpm for 10 mins and the chromium concentration in the supernatant was studied using atomic absorption spectroscopy. SEM studies revealed Sunflower stem has large surface area for binding of large number of adsorbates.8
Table 1: Comparison of various biosorbents
|
Biosorbent |
Metals |
Adsorption capacity |
Reference |
|
Sacchromyces |
Cu |
96% |
Manuels et al., |
|
cerevisiae |
Ni |
|
2007 |
|
|
Zn |
|
|
|
|
Cd |
|
|
|
A.niger |
Cd |
7.06 mmol/g |
Paula marques 2006 |
|
|
Cu |
97.5% |
Wan Xia Ren 2009 |
|
|
Cd |
88.2% |
|
|
|
Pb |
26 |
|
|
|
Zn |
14.5 |
|
|
Arthrobacter sp |
Cd |
33% |
F.Pagnaelli 2003 |
|
R.oryzae |
Cu |
|
|
|
Living |
|
19.4mg/g |
|
|
Non living |
|
43.7mg/g |
Kuber 2007 |
|
Mucor rouxii |
Pb |
17 |
Yan 2003 |
|
|
Zn |
4.89 |
|
|
|
Cd |
6.94 |
|
|
|
Ni |
5.74 |
|
|
P.chrysogenum |
Cd |
|
Skowruski 2001 |
|
Phanerochaete chrysosporium |
Pb |
2 |
Say etal |
|
Chlorella vulgans |
Cd |
111mg/g |
Asku 2001 |
|
Scenedemus incrassatula |
Cr |
|
|
|
Olive pomace |
Cd(II) |
0.100mmmollg |
H. Gao, |
|
Reed |
Pb |
0.082mmmollg |
B. Southichak, |
|
Laminoria |
CD |
1.67 |
Yinghiu liu |
|
japonica |
Cu |
1.62 |
|
|
|
Zn |
0.91 |
|
|
|
Ni |
1.22 |
|
|
Gelidium |
Cu |
0.571 g-1 |
Vitar J.P. Vilar |
|
Fucus vesiculous |
Pb, Cu |
Model equation |
Y.N. Mata |
|
|
|
was arrived |
|
|
Candida albicans |
Pb |
833mg/g |
Zubyde beysul |
|
Yeast biomass |
Cu |
2.59mg/g |
Corneliu |
|
Aspergillus foetidus |
Cr |
2mg/g |
Prasanjit 2005 |
|
Aureobasidium pullalans |
Cu |
18 mg/g |
Ahluwalia 2003 |
|
Cladosporium resinae |
Cu |
24mg/g |
Ahluwalia 2003 |
|
Pleurotus sapidus |
Hg |
127mg/g |
Skowronski 2001 |
|
R.oligosporus |
Cr |
126mg/g |
Ariff etal 1999 |
|
Aphanothece halophytica |
Zn |
133mg/g |
Incharoenskadi 2002 |
|
Dunaliella |
Cr |
58.3 |
Domenz and asku 2002 |
|
Pachymenopis |
Cr |
225mg/g |
Lee 2000 |
|
Bacillus firms |
Pb mg/g |
467 mg/g |
Salehizadeh 2003 |
|
B.coagulants |
Cr |
30.7 |
Srinath 2002 |
|
B.megaterium |
Cr |
39.9 |
Srinath 2002 |
Bael Fruit
Bael fruit was dried to 110°C in hot air oven and and reduced to size range of 600-800 µm. Chemical activation was done with 88% orthophosphoric acid. Batch kinetic study was performed for various pH range from 1-8 and various chromium concentration (50-125mg/L) with a predetermined time of 240 min in a orbital shaker. The batch studies were conducted by varying one parameter at a time. The samples were filtered using 42 number whatman filter paper and analyzed in a flame absorption spectrophotometer. Maximum removal of chromium was obtained at pH2 and the removal efficiency decreased 91.9% to 85.9 with increase in initial concentration of chromium from 50-125mgL-1.9
Wheat Straw
Cd and Cu removal b wheat straw was studied with various pH from 4 to 7 and varying metal concentrations 130 and 60 % removal of cd and cu respectively was reported with the increase in pH from 4 to 7, temperature also was studied as a significant parameter. Experimental studies revealed maximum removal at 25 to 30°C. Langmuir and Freundlich isotherm suited well for Cd. The study proposes wheat straw as a low cost biosorbent for heavy metals removal.10
Microbial Biomass
Microbe’s posses a large number of polysaccharides in their cell wall which helps in accumulation of metal ions. This mechanism is similar to that of ion exchange method of removal heavy metal with the advantage that cost of biomass is cheaper and can be regenertated easily.
Table 2: Various reactors for biological removal of metals
|
Bioreactor |
Metal |
Total Feed |
Total Removed |
Reference |
|
Airlift |
CdCr |
32.01mmol |
25.02mmol |
P.Marques 2007 |
|
|
|
18mg/l |
6mg/l |
|
|
Fixed bed |
Cd |
3.39mmol |
1.14mmol |
P.Marques 2007 |
|
Membrane reactor |
Cu |
1.5mmol |
0.5mmol |
F.Pagnelli |
|
Biocolumn reactor ( GAC) |
As |
12.5 ppm |
12.4ppm |
P.Mondal 2007 |
|
Batch reactor GAC |
As |
|
8% |
P.Mondal 2007 |
|
(Ralstonia eutropha) |
|
|
|
|
|
Soil bioreactor(97% of Cr |
Cr |
4320mg |
127mg |
Jeya Singh 2004 |
|
VI was reduced to Cr III which |
|
|
|
|
|
is less toxic compared with CrVI) |
|
|
|
|
|
Bench scale column |
Cr |
Monod Inhibition constant was derived |
T.Shasidhar 2006 |
|
Algae
Chladophora albida was investigated as a potential sorbent for chromium removal in batch experiments.11 The biomass was prepared by washing the algae with distilled water and oven drying at 60°C. Chromium removal rate was found to increase with increase in temperature and decrease in pH. The optimum pH was found to be Results for removal of chromium with algae were100% (28.09mg L-1 initial concentration) efficient and the author has emphasized on the regeneration of column as a scopeful future study.
Scenedesmus incrassatulus was studied for the removal of chromium. The micro alga was cultivated in a agar- nitrate modified basal medium. The well grown colony was picked and used for inoculating the photobioreactor. Culture conditions were as follows: temperature, 25 ± 2°C; air flow rate, 997 ml min”1. Cr solution was added into the photobioreactor up to 1 mg Cr(VI) l-1. Cr concentration in the feeding medium was verified every 3 d using an atomic absorption spectrometer. The algal suspension was filtered through a Millipore filter paper and the concentration of chromium was measured using atomic absorption spectrometer.43% removal efficiency was reported.12
Bacteria
Bacteria may uptake and accumulate a significant amount of metal ions resulting in the transfer of metal ions to a matrix. Suspensions of dead biomass of actinomycetes from industrial fermentation was mixed with waste water the biosorption of Cd occurred due to negatively charged sites in the bacterial cells.13 Arthrobacter sp was protanated with 0.1 N HCl and titrated with 0.1 NaOH. Biosorption tests were conducted for Cu and cd removal at different pH 4- 5. Subsequent addition method was employed (SAM) was employed. The equilibrium time for each metal was calculated as 30 min.
Fungal Biomass
Cu uptake by R. oryzae in Erlenmeyer flask containing 25 mL of Cu solution was studied in an environmental shaker at 150 rpm. The performance of biomass was found to be maximum at pH 4- 6. Alkali treatment (NaOH) of the biomass resulted in higher amount of Cu removal as alkali neutralized the protons in the native biomass making more binding sites available for Cu ions.14 Nonliving waste biomass of A. niger attached to wheat bran was used as biosorbent for removal of Zn and Cu from aqueous solution and metal uptake was found to be a function of the initial metal concentration, biomass loading and pH. Alkali treatment of A. niger biomass was found to sequester Cd, Cu, Zn effectively to 10% of its weight. Neurospora, Fusarium and Penicillium was also studied but A. niger gave better results in removal of Cd, Cu,Zn. Dry cells of R. arrhius were used for removal of Fe, Pb, Cd ions from waste water. Higher adsorption rate and adsorption capacity was obtained in a batch reactor. The maximum adsorption rate obtained was 100-150mgL-1 at pH 5 and 30°C.
Bioremediation
Sacchromyces cerevisiae was immobilized by entrapment in a matrix of polysulfolane. A fixed bed reactor and an airlift reactor were compared for their effective removal of Cd from dilute aqueous solutions. A liquid recirculation flow rate of 0.027. dm-3 min-1 in fixed bed reactor and an air injection flow rate of 0.1vvm was studied. Maximum removal of Cd was obtained in both reactor at pH 4-5. Airlift reactor gave superior results of 7.06 mmol Cd g biomass-1.4 This result gives an idea that bioremoval of metal can be done in continuous reactor arrangements.
Removal of chromium using a suspended growth system and an aerobic and anoxic attached growth system was done using Arthobacter rhombi.16 Synthetic wastewater was employed for the study purpose. The results obtained were encouraging for the use of bioreactors for metal removal. The aerobic suspended growth system resulted in 95% removal of chromium (20 mg/mL initial concentration). Further biokinetic parameters were also studied.16 The authors have presented a biokinetic equation for metal removal using MATLAB.
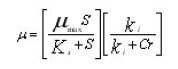
ki the inhibition constant, µmax is the maximum specific growth rate, µis the specific growth.
Conclusion
Metals can be removed over broad range of pH, temperature and initial concentration. The cost of the process is lesser compared to other techniques. Low concentration of heavy metals in effluents can be easily and effectively removed using biosorbents. Literature review reveals that a particular biosorbent can be used to remove a wide range of metals. Fungi and algae are found to have more metal removal capacity than bacteria. Biological sorbents are applicable all kinds of industrial effluents, the biggest advantage is that their ability to remove heavy metals from effluents containing low concentrations of metals. The use of immobilized microbes rather microbes in free nature is recommended as immobilized microbes has the advantage of withstanding high stress and metal load. A hybrid system consisting of a microorganism attached on to a support and its usage in a reactor is recommended as the specific condition required for bioremediation can be maintained inside a bioreactor thereby enhancing efficient removal.
References
- Kaustubha Mohanty, Mousam Jha B.C., Meikap M.N. and Biswas. Biosorption of Cr (VI) from aqueous solutions by Eichhornia crassipes. Chem. Engg Journal. 117: 71-77 (2006).
- Brierley C.L. Bioremediation of metal contaminated surface and groundwaters. Geomicrobiol. J. 8: 201-223 (1990).
- Shah K. and Nongkynrih J.M. Metal Hyperaccumulation and Bioremediation. Biologica Planatarium 51(4): 618-634 (2007).
- Paula Marques and Helena Mariam pinhero. CdII removal from aqueous solutions by immobilized waste brewery yeast in fixed bed and airlift reactors. Desalination 214: 343-351 (2007).
- Manuela D., Machado, Monica S., Santos F., Clauda Gouviea, Helena M.V. and Soares, Removal of heavy metals using Brewer’s yeast strain Sacchromyces Cerevisiae:The flocculation as a separation process. Bioresource Technology. 99: 2107-2115 (2008).
- Bounheng Southichak, Kazunori Nakano, Munehiro Nomura, Nobuo Chiba, Osamu Nishimura and Phragmites australis. A novel biosorbent for the removal of heavy metals from aqueous solution. Water Research. 40: 2295-2302 (2006).
- Tarun Kumar Naiya, Pankaj Chowdhury, Ashim Kumar Bhattacharya, and Sudip Kumar Das. Saw dust and neem bark as low-cost natural biosorbent for adsorptive removal of Zn(II) and Cd(II) ions from aqueous solutions. Chemical Engineering Journal. 148, 68–79 (2009).
- Monika Jain, V., Garga, K. and Kadirvelu K. Chromium (VI) removal from aqueous system using Helianthus annuus (sunflower) stem waste. Journal of Hazardous Materials. 162: 365-370 (2009).
- Srinath, T., Verma, T., Ramteke, P.W. and Garg, S.K. Chromium (VI) Biosorption and bioaccumulation by chromate resistant bacteria. Chemosphere. 48: 427-435 (2002).
- Anandkumar J. and Mandal B. Removal of Cr (VI) from aqueous solution using Bael fruit (Aegle marmelos correa) shell as an adsorbent. Journal of Hazardous Materials 162: 276–280 (2009).
- Dang V.B.H. Doan H.D. Dang-Vu T. and Lohi A. Equilibrium and kinetics of biosorption of cadmium (II) and copper (II) ions by wheat straw. Bioresource Technology 100: 211-219 (2009).
- Liping Deng, Yang Zhan, Jie Qin, Xinting Wang, Xiaobin Zhu Biosorption of Cr (VI) from aqueous solutions by nonliving green algae Cladophora albida. Minerals Engineering 22: 372-377 (2009)
- Carlos Rodrigo Jácome-Pilco, Eliseo Cristiani-Urbina, Luis Bernardo Flores-Cotera, Roberto elasco-García, Teresa Ponce-Noyola, Rosa Olivia, and Cañizares-Villanueva. Continuous Cr (VI) removal by Scenedesmus incrassatulus in an airlift photobioreactor, Bioresource Technology. 100: 2388-2391 (2009).
- Butter, T. J., Evison, I. M., Hancock., K, I. C., Holland, F. S., Matis, K. A., Philipson, A., Sheikh, A. I. and Zouboulis, A. I. The removal and recovery of cadmium from dilute aqueous solutions by biosorption and electrolysis at laboratory scale. Bioresource Technology. 100: 2335–2340 (2009).
- Ahluwalia, S.S. and Goyal, D. Removal of lead from aqueous solution by different fungi. Indian Journal of Microbiology. 43(4): 237-241 (2003).
- Elangovan and Ligy Philip. Performance evaluation of various bioreactors fpr the removal of Cr (VI) and organic matter from industrial effluent, Biochemical Engineering Journal. 44: 174-186 (2009).






