Eudrilus eugeniae as a putative candidate for textile industry effluent polluted soil bioremediation
A. Veeramani1 , S. Senthil Kumar2 , M.S. Mohamed Jaabir2 * , C. Sivagandhi2 , R. Marimuthu2 and R. Ravikumar3
DOI: http://dx.doi.org/10.12944/CWE.5.1.21
Efforts were taken to understand the impact of textile effluent on the population of earthworms mimicking the condition prevalent in Textile belt of Tamil Nadu where effluents are drained into the cultivable land through the water stream. Categories of earthworms were maintained and drained with calculated amounts of raw, chemically and biologically treated effluents for a period of 8 weeks in which fecundity and growth were recorded. There was significantly high fecundity in the soil treated with raw effluent than that of control and the treated effluents suggesting us to use of these worms for bioremediation of textile effluent polluted soil.
Copy the following to cite this article:
Veeramani A, Kumar S. S, Jaabir M. S. M, Sivagandhi C, Marimuthu R, Ravikumar R. Eudrilus eugeniae as a putative candidate for textile industry effluent polluted soil bioremediation. Curr World Environ 2010;5(1):131- 136 DOI:http://dx.doi.org/10.12944/CWE.5.1.21
Copy the following to cite this URL:
Veeramani A, Kumar S. S, Jaabir M. S. M, Sivagandhi C, Marimuthu R, Ravikumar R. Eudrilus eugeniae as a putative candidate for textile industry effluent polluted soil bioremediation. Curr World Environ 2010;5(1):131- 136. Available from: http://www.cwejournal.org?p=257/
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2010-04-12 |
|---|---|
| Accepted: | 2010-06-17 |
Introduction
Textile industries consume large amount of water (60-4001/kg of fabric) and chemicals for wet processing.1 The chemical reagents used in textile sector are diverse in chemical composition ranging from inorganic to organic. The inputs of wide range of chemicals, which, if not incorporated in the final products (fabric), become waste and turn out to be part of water ecology. Generally, textile effluent is colored, varying in hydraulic flow rate, having high; pH, temperature, biological oxygen demand (BOD), chemical oxygen demand (COD), total dissolved solids (TDS) and total suspended solids (TSS).2-4
Color is imparted to textile effluents because of various dyes and pigments used. Many dyes are visible in water at concentrations as low as 1 mg/L. textile wastewaters, typically with dye content in the range of 10-200mg/L are therefore highly colored. In addition to this, various salts and chemicals are major sources of heavy metals in wastewater.5 Sediments, suspended and dissolved solids are important repositiores for toxic heavy metals and dyes6,7 causing rapid depletion of dissolved oxygen leading to oxygen sag in the receiving water.8
The key environmental issues associated with textile manufacture are; water use, treatment and disposal of aqueous effluent. Textile effluents are mostly discharged after minimal or no pretreatment into the adjoining water channels, streams and estuaries.9,10
There is a growing emphasis on biological remediation associated with their cost effective and long lasting nature. Presently, textile belt of Tamil Nadu as popularly called had been draining their effluents into the streams and waste lands that were once cultivable until strict rules and regulations were passed to confine their effluents. Since then, the industries have been treating their effluents either by chemical treatment or using biological agents such as bacterium or fungi. However, the large quantities of water ultimately reach the ecosystem even after careful recycling and purification processes as being done recently. Since earthworms are major components in the cultivable lands where the textile industry polluted waters drain into, a study has been undertaken to record the impact of these textile dye industry effluents (raw and chemical or biologically treated) on the population of these worms. Eudrilus eugeniae was employed for the purpose.
 |
Figure 1: Soil and cowdung mixed in the ratio of 1:1 (1.a); Basket with many perforations to allow aeration of the soil (1.b); Experiments conducted in triplicates (1.c); Earthworms maintained in baskets receiving tap water (control Group I), raw effluent (Group II), chemically treated (Group III) and biologically treated effluent (Group IV) (1.d) Click here to view Figure |
Material and Methods
Collection of Raw, Chemically and Biologically Treated Textile Dye Effluent
Textile dye effluents were collected from the United Bleachers Limited at Mettupalayam separately as raw effluent, chemically treated effluent and biologically treated effluent. These were brought to the lab and used for the restoration of moisture content in the earthworm medium as per the treatment regime.
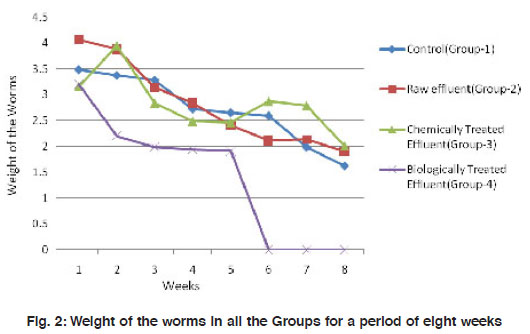 |
Figure 2: Weight of the worms in all the Groups for a period of eight weeks Click here to view figure |
Collection of Earthworms, Treatment and their Maintenance
Earthworm, Eudrilus eugeniae were collected from the Periyar Research Organization for Bio-Technique & Eco-System (PROBE), Periyar Maniyammai University, Vallam, Tanjore Dist, Tamil Nadu, South India. Organic waste served as a medium of growth for the worms in the sieved garden soil and cow dung mixed in the ratio of 2:1. The mixture was allowed to dry under sun-light for 10 hours. After mixing subsequent amount of water, the worms were allowed to be contained in suitable perforated plastic containers with the soil (Fig.1a, 1b). The study was carried out in four groups such as the Control (Group I) receiving only tap water, Group II: receiving raw effluent, Group III receiving the chemically treated effluent and Group IV receiving the biologically treated effluent(Fig.1c,1d).. The effluents were given for the first ten days followed by the tap water for all the groups in order to accommodate study in a small volume of soil taken in the culture-basket. The study was prolonged for a period of 8 weeks with a changeover of only 30% of medium (wt/wt) at the end of 4 weeks. Care was taken to rear the worms with 82% of moisture in the immediate surroundings. The optimum temperature was maintained constantly by spreading some banana leaves over the culture basket. Viability of the worms, cocoon production, hatchlings and weight of the worms were recorded for every seven days from day 1. At the time of recording, the worms were taken out gently in a cotton mesh and washed with a gentle flow of water. For this, the soil from each basket was carefully placed on a clean white surface and the worms, cocoons and hatchlings were collected carefully and weighed or counted as appropriate.
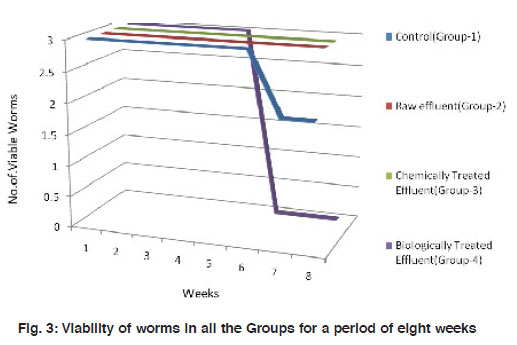 |
Figure 3: Viability of worms in all the Groups for a period of eight weeks Click here to view figure |
Results and Discussion
Number and weight of the worms, number of hatchlings and cocoons were recorded at an interval of seven days for 8 weeks in all the treatment groups. Viability of the worms were recorded for a period of 8 weeks where, worms in Group II and the Control (Group I) were all viable. Group III and Group IV worms demonstrated mortality to 33% and 100 % respectively at sixth week (Fig. 2). Absolute mortality observed in Group IV worms is possibly due to the odour, heavy microbial load and/or the metabolites present in the biologically treated effluent.
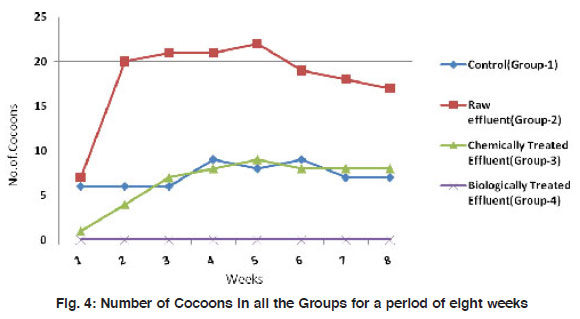 |
Figure 4: Number of Cocoons in all the Groups for a period of eight weeks Click here to view figure |
The mean weight of the worms have reduced significantly (p<0.001) from 3.48±0.92 to about 1.65±0.65 in the control group I. A similar observation was made in the treatment groups II and III also where there is a reduction in size of worms by 2-3 folds. However, in the biologically treated group (IV), there was high mortality after 5 weeks (Fig. 3). In Group IV, in week 5, the weight of the worms reduced to nearly 3 folds when compared to the control which took 8 weeks for similar observation. All the three worms in the biologically treated effluent-soil died in the sixth week itself. As a measure of the population growth, cocoon production was recorded among all the three treatments in comparison with the control. An average of 7 cocoons were seen in the control group whereas, in the group-II (raw effluent) it was raised to a maximum of 22 in the fifth week and finally were 17 at the end of eight weeks. This is a significant increase when compared to the control group-I (Fig. 4). In the chemically treated effluent-soil, the number of cocoons was comparable to that in the control without any significant change. In contrast to this, there were no cocoon productions seen in the biologically treated-soil group (IV). Hatchlings were counted every seven days and there were two hatchlings at the end of four weeks in the control group-I which was significantly (p<0.001) different from the (Group II) raw effluent treated-soil which produced 7 cocoons. Chemically treated and biologically treated-soil groups (III and IV) did not produce hatchlings through cocoons (Fig. 5). There was significant loss in weight of the worms throughout the experimental period which is probably due to the limited space and soil available in the basket containing the soil. Chemically treated effluent-soil in group-III demonstrated stress and toxicity due to chemicals to certain levels that caused morbidity and mortality though not to the level of the biologically treated effluent-soil group-IV.
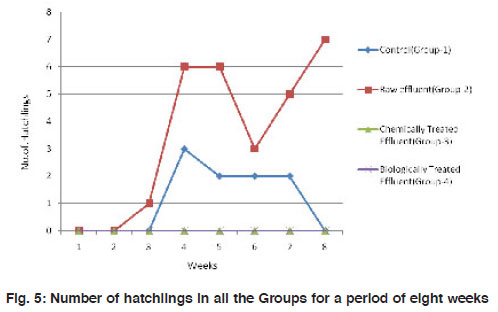 |
Figure 5: Number of hatchlings in all the Groups for a period of eight weeks Click here to view figure |
A greater proportion (>80%) of the biomass of terrestrial invertebrates is represented by earthworms which play an important role in structuring and increasing the nutrient content of the soil. Therefore, they can be suitable bio-indicators of chemical contamination of the soil in terrestrial ecosystems providing an early warning of deterioration in soil quality.11-13 This is important for protecting the health of the natural environment and is of increasing interest in the context of protecting human health14 as well as other terrestrial vertebrates which prey upon earthworms.15 The suitability of earthworms as bioindicators in soil toxicity is largely due to the fact that they ingest large quantity of decomposed litter, manure and other organic matter deposited on soil, helping to convert it into rich topsoil.16-17 Moreover, studies have shown that the earthworm skin is a significant route of contaminant uptake18 and thus investigation of earthworm biomarkers in the ecological risk assessment (ERA) can be helpful.19 They have also been observed to be valuable as index organisms in evaluating the impact of soil-borne contaminants.20-22 With the increasing rate of industrialization and urban development, there is no doubt that the environment we live in today is exposed to greater pollution. There is a need therefore to evaluate an off-side test for assessing the impact of textile industry effluent on the activities of the earthworm species Eudrilus eugeniae.
From the present investigations, it is clear that the earthworms are known not to suffer significant morbidity from exposure to raw textile industry effluent. Moreover, they seem to flourish with high fecundity in the presence of raw textile effluent than that of the control water and treated effluents. This is a unique and striking finding which is different from the expected outcome indicating us to conduct further studies on testing the putative employment of earthworms in the bioremediation of such soils and their uses thereof to promote growth, development and yield of a crop plant.
References
1. AEPA (Australian Environmental Protection Authority), Environmental guidelines for the textile Dyeing and Finishing Industry, State Government of Victoria, Melbourne, Victoria, Australia (1998).
2. Buckley, C. A. Membrane technology for the treatment of dyehouse effluents, Water Sci. Technol. (1992) 25(10): 203-209.
3. Banat, I.M, Nigam, P, Singh. D. and Marchant, R. Microbial decolorization of textile dye containing effluents, a review, Bioresour. Technol. (1996) 58: 217-227.
4. Ghoreishi, S. M. and Haghighi, R. Chemical catalytic reaction and biological oxidation for treatment of non-biodegradable textile effluent, Chem. Eng. J. (2003) 95: 163-169.
5. Wagner, S. Improvements in products and processing to diminish environmental impact, in: COTTECH Conference, Raleigh, NC, November (1993) 11-12.
6. Tamburlini, G, Ehrenstein, O.V. and Bertollini, R. Children’s health and environment: a review of evidence, Environmental Issue Report No. 129, WHO/European Environment Agency,WHO Geneva. (2002) 223.
7. Chapman, P.M, Romberg, G.P. and Vigers, G. A. Design of monitoring studies for priority pollutants, J.Water. Pollut. Control Fed. (1982) 54(3): 292-297.
8. Ademoroti-C, M. A, Ukponmwan, D.O. and Omode, A. A. Studies of textile effluent discharges in Nigeria, Environ. Stud. (1992) 39(4): 291-296.
9. Yusuff, R. O, Sonibare, J. A. Characterization of textile industry’ effluents in Kaduna, Nigeria and pollution implications, Global Nest: Int. (2004) 6(3): 212-221.
10. Robinson, T. McMullan, G. and Marchant, R. Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative, Bioresour. Technol. (2001) 77: 247-255.
11. Culy, M.D. and Berry, E.C. Toxicity of soilapplied granular insecticides to earthworm populations in cornfields. Down to Earth. (1995) 50: 20-25.
12. Sorour, J. and Larink, O. Toxic effects of benomyl on the ultrastructure during spermatogenesis of the earthworm Eisenia foetida. Ecotoxicol Environ Saf. (2001) 50: 180-188.
13. Bustos-Obregon, E. and Goicochea, R.I. Pesticide soil contamination mainly affectsearthworm male reproductive parameters. Asian J Androl. (2001) 4(3): 195-199.
14. Beeby, A. What do sentinels stand for? Environ Pollut. (2001) 112: 285-298.
15. Dell’Omo, G. Turk, A. and Shore, R.F. Review of Biological Proceedings. Environ Toxicol Chem. (1999) 18: 237-240.
16. Reinecke, S.A. and Reinecke, A.J. Lysosomal response of earthworms coelomocytes induced by long-term experimental exposure to heavy metals. Pedobiologia (1999) 43: 585-593.
17. Sandoval, M.C, Veiga, M, Hinton, J. and Klein, B. Review of Biological indicators for metal mining effluents: A proposed protocol using earthworms. Proceeding of the 25th Annual British Columbia Reclamation Symposium. (2001) 67-79.
18. Lord, K.A, Briggs, G.G, Neale, M.C. and Manlove, R. Uptake of pesticides from water and soil by earthworms. Pestic Sci. (1980) 11: 401-408.
19. Sanchez-Hernandez, J.C. Earthworm biomarkers in ecological risk assessment. Rev Environ Contam Toxicol. (2006) 188: 85-126.
20. Ireland, M. P. Metal accumulation by earthworms Lumbricus rubellus, Dendrobaena veneta and Eisenia tetraedra living in heavy metal polluted sites. Environ. Pollut., (1979) 13: 66-69.
21. Callahan, C. A, Menzie, C.A, Burmaster, D.E, Wiborn, D.C. and Erns, T. On site method for assessing chemical impacts on the soil environment using earthworms. A case study at the Baird and McGuire superfund site. Environ. Toxicol. Chem., (1991) 10: 817-826.
22. Schaefer, M. Earthworms in crude oil contaminated soils: Toxcity tests and effects on crude oil degradation. Environ. Asses. Remed., (2001) 8: 35-37.






