Esterification of phthalic anhydride with n-Butanol using eco-friendly solid acid catalyst-sulfamic acid
M.S. Patil1 * , V.D. Gurudasani1 and G.A. Usmani2
DOI: http://dx.doi.org/10.12944/CWE.5.1.16
Present study involves investigation of Sulfamic acid as a catalyst for esterification of Phthalic anhydride with n- Butanol. Effect of different operating parameters such as molar ratio of reactants, catalyst quantity and operating temperature has been studied with an aim of optimization. The optimum parameters for this process have been found to be 1:.2 molar ratio of Phthalic anhydride to n-butanol, catalyst concentration of 6%(by weight) of reaction mixture and 130-180 degrees C reaction temperature. Maximum conversion levels of about 89% have been obtained in 1.5h of reaction time under these optimized conditions.
Copy the following to cite this article:
Patil M. S, Gurudasani V.D, Usmani G. A. Esterification of phthalic anhydride with n-Butanol using eco-friendly solid acid catalyst-sulfamic acid. Curr World Environ 2010;5(1):107-109 DOI:http://dx.doi.org/10.12944/CWE.5.1.16
Copy the following to cite this URL:
Patil M. S, Gurudasani V.D, Usmani G. A. Esterification of phthalic anhydride with n-Butanol using eco-friendly solid acid catalyst-sulfamic acid. Curr World Environ 2010;5(1):107-109. Available from: http://www.cwejournal.org/?p=1116
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2010-02-18 |
|---|---|
| Accepted: | 2010-04-10 |
Introduction
Sulfamic acid is strong mineral acid existing in crystalline form .It is cheap. It is used to clean equipments and vessels in food industry to remove deposits, scales and oxide films .It is also used to sulfonate organic substances like alcohol, phenol etc. As catalyst it can replace sulfuric acid which is widely used for esterification. Use of sulfamic acid as a catalyst for esterification has many advantages over sulphuric acid and other catalyst. Aim of present work was to investigate the use of sulfamic acid as a catalyst for esterification of phthalic anhydride with n-butanol to produce dibutyl phtalate.Dibutyl phthalate has wide industrial application. One of which is as a plasticizer in paint industry
Experimental
Phthalic anhydride was esterified with butyl alcohol (n-butanol) using Sulfamic acid as a catalyst. Total 7 batches of above system were taken. First two batches were taken at two different sulfamic acid concentrations viz, 3% & 6% of total weight of reaction time & conversion. Mole ratio of phthalic anhydride & n-Butanol was kept constant at 1:2.5 in both batches.
A three naked flat bottom one liter capacity flask was used as a reactor. It was kept on rotamantle provided with heater & magnetic stirrer. One neck was provided with thermometer well. Middle was stoppered .Third neck was connected to Dean and Stark apparatus provided with reflux condenser.
n-butanol & phthalic anhydride both were L.R. Grade, of above 99% Purity & manufactured by Loba chemi Private Limited. Sulfamic acid – Commercial grade supplied by M/S P J Chemicals.
Into the flask was charged a 185 gm n-butanol (2.5mols) & 148 gm phthalic anhydride (1mo1) . Heating & stirring was started to dissolve phthalic anhydride in to n-Butanol. After dissolving the phthalic anhydride into n-butanol. 10 gm of sulfamic acid was added into the reactor.
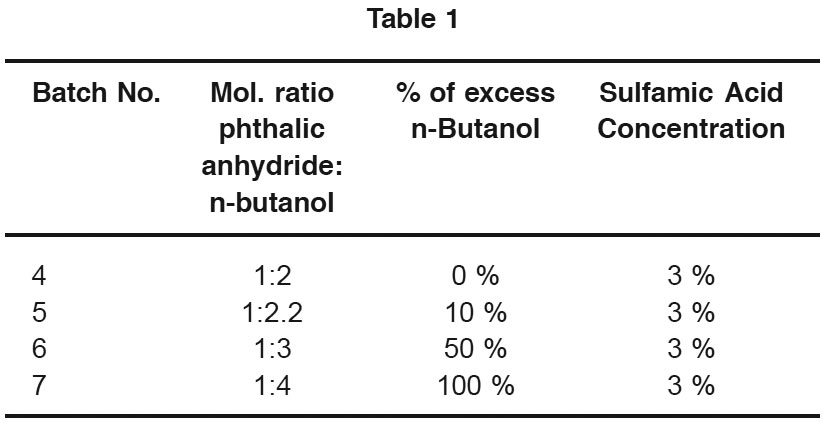 |
Table 1 Click here to view table |
The course of reaction was followed by the quantity of the water collected in the separator. For 100% conversion of 1mo1of Phthalic anhydride, quantity of water collected should be 18 ml, However it was found that after getting a 15 ml of water, the rate of evolution of water decreased considerably. It was found that some water was with sulfamic acid. The reaction was considered as completed. The reaction product was cooled to room temperature. Sulfamic acid was then filtered off.
Reacting mass was taken into 1 L beaker, to this raw water was added. Mixture was then stirred at room temperature for 4-5 minute .The heterogeneous mixture was allowed to settle into two layers in the separating funnel. Lighter aqueous layer was discarded. Heavier oil layer was subjected to rewashing as described above. Purpose of washing was to remove the traces of sulfamic acid and unreacted phthalic anhydride.
Washed product was then subjected to steam distillation to remove the unreacted n-butanol. 10 gm of sodium carbonate was added to washed product before steam distillation. Stem distillation was carried out at atmospheric pressure. Steam was produced in pressure cooker (2 L volume). The course of steam distillation was followed by smelling the distillate. Steam distillation was stopped, when no smell of alcohol was found in distillate. Steam distillated product which contains condensed steam was allowed to separate into two phases.
 |
Table 2 Click here to view table |
Both phases were separately received. Aqueous phase was discarded. The oil phase which is final product free from Sulfamic acid, phthalic anhydride and n-butanol, was weighted. Purity of final product was determined by measuring the Refractive index and Specific Gravity of final product. These properties for pure dibutyl phthalate, (DBP) are Refractive index. - 1.491, Specific Gravity - 1.04
The catalyst used in batch 2, was reused in batch 3. The quantities of phthalic anhydride and n-Butanol were same as that in Batch2. Experimental procedure was same as explained above.
Four batches were taken at different mol ratio of phthalic anhydride & n-Butanol. Moles of phthalic anhydride and concentration of catalyst was kept constant in each of the four runs. Following mole ratios of phthalic anhydride & n-butanol were taken.
Results and Discussion
Ester obtained in each batch was almost pure. Refractive index was,1.491-1.492 , Specific Gravity was 1.045-1.048
Table 2 shows that with increase in concentration of the catalyst the time of reaction decreases. Generally it is found that the rate of acid catalyzed esterification is proportional to acid or hydrogen ion concentration. The result is in agreement with above statement.
It can be seen from Table II that the reaction temperature has decreased with increase in % of excess alcohol. Increase in % of alcohol in reaction mixture decreases its boiling point, since rate of reaction is proportional to the tempature, the reaction time is more for bathes containing higher % of excess alcohol. In present work it is found that maximum conversion levels of about 89% have been obtained in 1.5h of reaction time under 1:.2 molar ratio of feed and 6% (by weight of reaction mixture) of catalyst.
Conclusion
The result of present work indicates that the sulfamic acid is an effective catalyst for esterification of the Phthalic Anhydride with n-Butanol. Result indicates that sulfamic acid can replace sulphuric acid or other mineral acids in esterification process.
Since sulfamic acid is less corrosive to metals than the sulfamic acid, less corrosion allowance is required while designing the equipments for esterification by sulfamic acid. Hence initial cost of the equipments for esterification by sulfamic acid is less than that by sulphuric acid. Process of esterification by sulphuric acid involves costlier waste treatment of acidic washing. Washing is treated with alkali NaOH or Na2Co3. Recovery of resulting salt is uneconomical due to its low cost. On other hand, catalyst sulfamic acid which is insoluble in most of organic liquids, settles at the bottom of the reactor after cooling of the reaction product. It can be removed from reaction product by simple filtration and then reused.
References
- V.V.Moslva, N I Grishko, N .I. Artamanova and T.B. Muravalyanskaya, ‘ Kinetika I kataliz ’ (1971) 12 , 1569.
- Japan pat 70674338. Ando sumio and nalmato Takao (1970).
- Rom pat .59087 Mongiuca Avram (1969).
- Balozina L .M, Nugatkina G.I and Kutsenko A .l ‘ Khim Prom st ’ (1978) 580(8).
- Nowokowski, lech and kaledkowska, mulgortala ‘Chem.Engg. Tech.’ (1986) 58, 48.
- O.M.Sharma, G.D.Nageshwar and P .S. Mene ‘Ind Journal of Tech.’ (1973) 11, 360.






