Polarographic reduction of pralidoxime and obidoxime at hanging mercury drop electrode
C. Narsimha Rao1 * , K. Balaji1 , C. Narsimha Rao2 and P. Venkateswarlu1
DOI: http://dx.doi.org/10.12944/CWE.4.1.20
The polarographic reduction behavior of Pralidoxime (PRL) and Obidoxime (OBD) at a Hanging Mercury Drop Electrode (HMDE) was exploited for their determination in different samples. Based on the obtained differential pulse polarograms, standard addition method was used to determine these drugs in pharmaceutical formulations and biological fluid samples. Linearity in the peak currents was achieved in the concentration ranges of 5.4 x 10-8 to 4.0 x 10-5 M and 2.8×10-8 to1.4×10-5 M for OBD and PRL respectively.The detection Limit was found to be 2.5 x 10-8 M (PRL) and 1.8×10-8 M (OBD) with correlation coefficients of 0.9980 (PRL) and 0.9965 (OBD). The repeatability and reproducibility of the method were checked by recovery studies.
Copy the following to cite this article:
Rao C.N, Balaji K, Rao C.N, Venkateswarlu P. Polarographic reduction of pralidoxime and obidoxime at hanging mercury drop electrode. Curr World Environ 2009;4(1):133-136 DOI:http://dx.doi.org/10.12944/CWE.4.1.20
Copy the following to cite this URL:
Rao C.N, Balaji K, Rao C.N, Venkateswarlu P. Polarographic reduction of pralidoxime and obidoxime at hanging mercury drop electrode. Curr World Environ 2009;4(1):133-136. Available from: http://www.cwejournal.org/?p=913
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2009-02-10 |
|---|---|
| Accepted: | 2009-04-13 |
Introduction
Pralidoxime (2-[(hydroxyimino) methyl]-1-methylpyridin-1-ium) (PRL) and Obidoxime (1, 1'-[oxybis (methylene)]bis{4-[(E)- (hydroxyimino) methyl] pyridinium) (OBD) are used to combat poisioning by organophosphates. Azomethine group containing drugs have been in wide use because of their pharmacokinetic properties (1-3). Several researchers have reported the determination of azomethine group containing drugs. (4-10). Determination of PRL was carried out by HPLC (26). Spectrophotometric (29) and Potentiometric (30) methods.Determination of OBD was carried out by HPLC (32, 33) and Spectrophotometric (35) methods. In the present method, a simple, accurate and cheaper method has been described for the determination of PRL and OBD.
Experimental
Voltammograms were recorded with Metrohm 757 VA computrace (Herisau, Switzerland).Pralidoxime and obidoxime were purchased from Cipla labs India Ltd., (Mumbai). Standard stock solutions (1.0X10-3 mol l -1) are prepared by dissolving an appropriate amount of electroactive species in deionised triple distilled water.
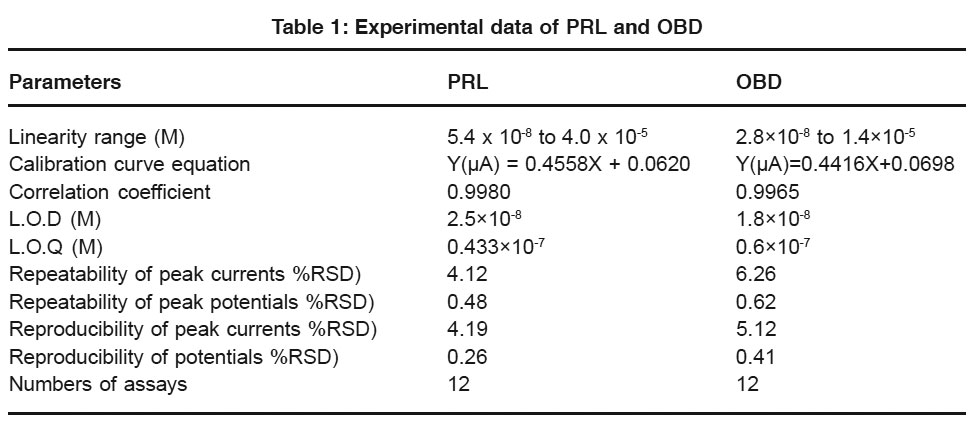 |
Table 1: Experimental data of PRL and OBD Click here to view table |
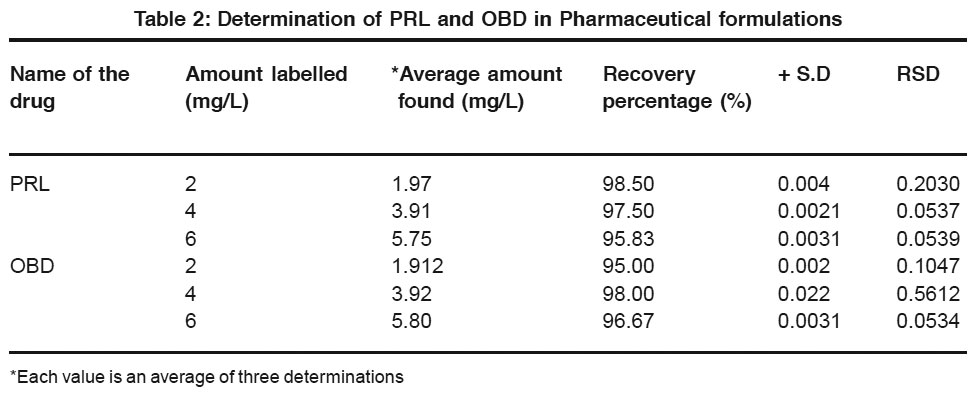 |
Table 2: Determination of PRL and OBD in Pharmaceutical formulations Click here to view table |
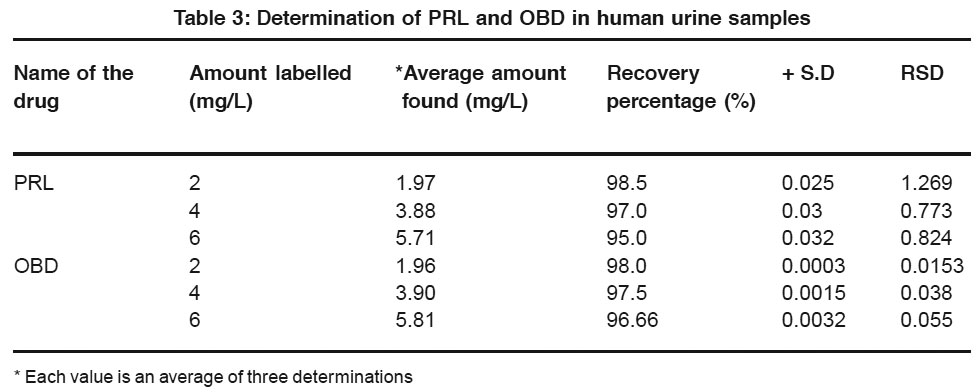 |
Table 3: Determination of PRL and OBD in human urine samples Click here to view table |
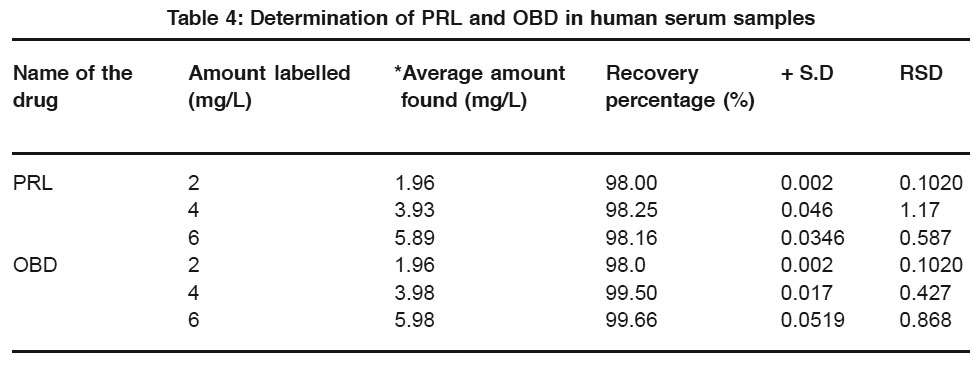 |
Table 4: Determination of PRL and OBD in human serum samples Click here to view table |
Recommended Analytical Procedure
Ten milli liters of BR buffer solution was deoxygenated in the cell with nitrogen gas. An aliquot of standard solution of the electroactive species was added to the buffer present in the cell. After recording the polarograms small increments (0.2 mL) of standard solution were added and polarograms were recorded after each addition under the same conditions.
Results and Discussion
Cyclic Voltammetry
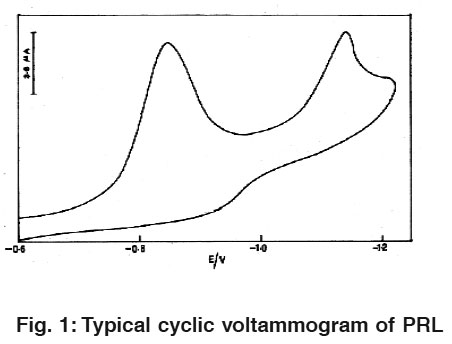 |
Figure 1: Typical cyclic voltammogram of PRL Click here to view figure |
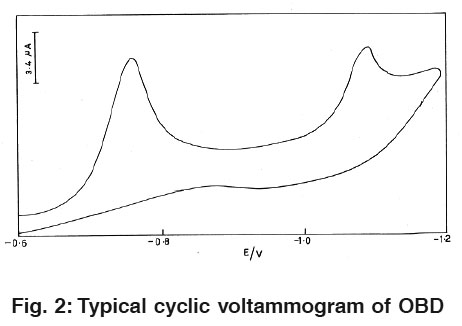 |
Figure 2: Typical cyclic voltammogram of OBD Click here to view figure |
Fig.1 and 2 Illustrate cyclic voltammograms (CV) of 1.6×10-8 M pralidoxime and obidoxime in 0.04M BR buffer solution of pH 2.0 at HMDE. On scanning from -0.4 to -1.4 v towards a negative potential two cathodic peaks are observed which are attributed to the reduction of azomethine group.
Differential Pulse Polarography
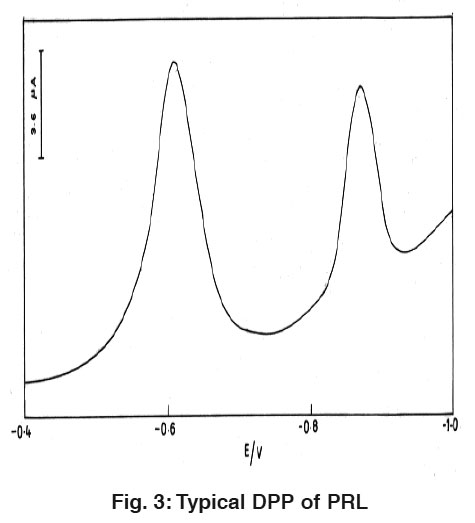 |
Figure 3: Typical DPP of PRL Click here to view figure |
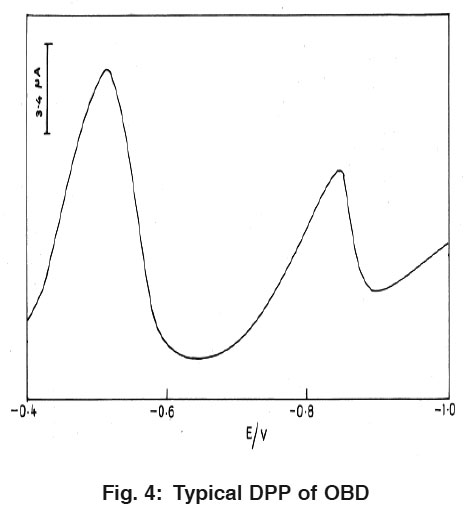 |
Figure 4: Typical DPP of OBD Click here to view figure |
Fig. 3 and 4 explain the differential pulse polarogramms for 1.6×10-8 M PRL and OBD in 0.04M BR buffer solution of pH 2.0 at HMDE. While scanning towards cathodic direction two peaks were observed and no peak in anodic direction indicating the irreversible nature of reduction process. The peaks are attributed to the reduction of azomethine group.
Conclusion
From the experimental results obtained, PRL and OBD are found to give two well-defined peaks in the BR buffer solution of pH 2.0 which are attributed to the reduction of azomethine group.Standard addition method is employed for the estimation of these pharmacologically important drugs in their pharmaceutical formulations, serum samples and urine samples.
References
2. P. Garzone, J.A. Lyon and V.L. Yu, Drug Intell. Clin. Pharm., (1983). 17: 615
3. B. Van Klingeren, L. J. Van Wijngaarden and A. Rutgers, J. Antimicrob. Chemother, (1980) 674(6): 676.
4. G.V. Subba Reddy and S. Jayarama Reddy, Talanta, (1997) 44: 627.
5. T. Madhusudana Reddy, M. Sreedhar and S. Jayarama DDY, j. Pharm. Biomed. Anal. (2003) 31: 811.
6. B. Ogorevc, M.R. Symth, V. Hudnik and S. Gomiscek, Anal. Chem. Ser., (1986) 25: 403.
7. I.F. Jones, J.E. Page and C.T. Rhodes, J. Phar. Parmacol., (1968) 20: 455.
8. G.Dusinksky and P. Antolik, Cesk. Farm., (1967) 16: 120.
9. G. Dusinsky and P. Antolik, Cesk. Farm., (1967) 16: 461.
10. D.A. Hall, J. Pharm. Sci., 62: 980 (173).
11. Chadi Abbara, Isabelle Bardot, Annie Cailleux, Guy Lallement, Anne Le Bouil, Alain Turcant, Pascal Clair, Bertrand Diquet, J. Chrom. B, (2008) 874: 42-50.
12. M. Bodiroga, D. Agbaba, D. Zivanov-Stakic, R. Popovic, J. Pharm. Biomed. Anal., (1994) 12: 127-129.
13. Katarina, D. Karljikovic-Rajic, Branislava, S. Stankovic, Clin. Chim. Acta., (1990) 193: 119-124.
14. Ahmet C. Goren, Gokhan Bilsel, Mine Bilsel, Serpil Yenisoy-Karakas, Duran Karakas, J. Chrom. A, (2004) 1057: 237-239.
15. C. Grasshoff, H. Thiermann, T. Gillessen, T. Zilker, L. Szinicz, J. Chrom. B: Biomed. Sci. Appli., (2001) 753: 203-208.
16. K. Karljikovic-Rajic, B. Stankovic, Z. Binenfeld, J. Pharm. Biomed. Anal., (1987) 5: 141-149.






