Removal of Zn2+, Cu2+ and Ni2+ ions from aqueous solution via Tripoli: Simple component with single phase model
Tayel El Hasan2 , Zaid A. Al-Anber1 , Mohammad Al-Anber2 * , Mufeed Batarseh3 , Farah Al-Nasr4 , Anf Ziadat5 , Yoshigi Kato3 and Anwar Jiries2
1
Department of Chemical Engineering,
Faculty of Engineering Technology,
Al-Balqa Applied University, Amman,
P.O Box 15008,
Amman 11131
Jordan
2
Department of Chemical Science,
Faculty of Science,
Mutah University,
61710 Al-Karak,
P.O Box 7
Jordan
3
Prince Faisal Center for Dead Sea,
Environmental and Energy Research, Mu’tah University,
P.O. Box 3,
Karak 61710
Jordan
4
Faculty of Agriculture,
Mutah University,
Al-Karak,
P.O Box 7
Jordan
5
Faculty of Engineering,
Mutah University,
Al-Karak,
P.O Box 7
Jordan
DOI: http://dx.doi.org/10.12944/CWE.3.1.01
The removal of heavy metals (Cu2+, Zn2+ and Ni2+) from aqueous model solution has been studied using Tripoli (Microcrypto crystalline silica = MCCS) as adsorbent. The adsorption equilibrium studies are performed with a constant initial metal ion concentrations (namely 10 and 100 mg.L-1) and varying adsorbent weight. The adsorption percentages of Zn2+ and Cu2+ ions increase sharply by increasing adsorbent doses in the range of 0.5 g to 5 g (approx.) and then it slightly increases in the range of 5 g to 10 g of Tripoli, while the percentage removal of Ni2+ increases sharply in the whole range of 0.5 to 10 g of Tripoli. In this level, the maximum adsorption might be attained. The best pH value is at about 7 to achieve the maximum removal, otherwise the precipitation of sorbet and the hydrolysis of sorbent are occurred. The removal percentages at pH = 7 are high (Approx. above 92%), where the diluted Zn2+ is characterized as the highest removal efficiency. The obtained experimental data has successfully fitted to the Langmuir and Freundlich isotherm models. The Freundlich constant Kf for Zn2+ is greater than the other heavy metals for the initial concentration 100 mg.L-1. The negative value of DG° confirms the feasibility of the process and the spontaneous nature of adsorption with a high preference for metal ions (Zn2+, Cu2+ and Ni2+, respectively) to adsorb onto Tripoli
Copy the following to cite this article:
El-Hasan T, Al-Anber Z.A, Al-Anber M, Batarseh M, Al-Nasr F, Zaidat A, Kato Y, Jiries A. Removal of Zn2+, Cu2+ and Ni2+ ions from aqueous solution via Tripoli: Simple component with single phase model. Curr World Environ 2008;3(1):01-14 DOI:http://dx.doi.org/10.12944/CWE.3.1.01
Copy the following to cite this URL:
El-Hasan T, Al-Anber Z.A, Al-Anber M, Batarseh M, Al-Nasr F, Zaidat A, Kato Y, Jiries A. Removal of Zn2+, Cu2+ and Ni2+ ions from aqueous solution via Tripoli: Simple component with single phase model. Curr World Environ 2008;3(1):01-14. Available from: http://www.cwejournal.org?p=87/
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2008-03-21 |
|---|---|
| Accepted: | 2008-05-05 |
Introduction
Jordan is considering among the poorest ten countries in water resources (Deutsche Gesellschaft fur Technische Zusammenarbeit (GTZ), 2006). Thus the purification and re-utilization of waste and industrial water has been given maximum attention. Phosphate mine industry in Jordan usually produces a large amount of effluent water from ore upgrading process. Beside the need for this amount of water; it is considered one of the environmental pollution sources because it contains several kinds of contaminants particularly the heavy metals. These heavy metals in the large concentration scales are considering one from the most environmental and human health problems.1-4 These heavy metals start to influence on human health by increasing of the heavy metal concentrations over the standard and recommended levels for drinking water and food. The National Research Council (NRC) in 1977 has stated the limited standards cations concentration in the drinking water at about 1.0 mg/L, <50 µg/L and 5.0 mg/L for the copper, nickel, and zinc, respectively.5 The problem is due to: firstly, the high atomic densities of the heavy metals that usually associated with toxicity. Secondly, these metals are not biodegradable and tend to accumulate in living organism and then cause various diseases and disorders.6-7 More specifically, the excess concentration from some heavy metals in soils such as Cd2+, Cr6+, Cu2+, Ni2+ and Zn2+ cause the disruption of natural aquatic and terrestrial ecosystems.8-11 Therefore, the excess concentration of metal ions must be removed from municipal and industrial effluents before discharge.
Many alternative chemicals, processes, studies and traditional techniques12-23 were proposed for removing the heavy metals out of the waste effluent water14-16. In general, these techniques include ion exchange, precipitation, phytoextraction, ultra filtration, reverse osmosis, and electro-dialysis.24-27 Some of the examples are organic precipitants such as DTC, (alkyl dithiocarbamates), modified natural zeolites and membrane filtration (micro-, ultra- and nanofiltration).28-30 Their application in many practical situations is however limited, due to various interferences, development and control costs, maintenance problems and process limitations.
Generally, the ion exchange and sorption by using natural adsorbents are reported to be the potential alternative for removing the heavy metal ions from aqueous mixtures. Recently, these alternatives have been chosen due to the several reasons, for example, it has easy handling, low cost, and safe for the environment and in the same time have good adsorption properties.31-32 There are many publications deal with several kinds of natural adsorbents, but in the same time are not meet all effective adsorbent factors. Among these published adsorbents are mostly not cost effective or/ and have limiting resources, for instance using date-pits, spent Animal Bones and Keratin-composed biosorbents.33-45
Basing on the effective adsorbent factors and on our recent works in this field, Tripoli is chosen as one of the natural inorganic materials that could be used for treating the wastewater.46-48 Due to several factors and conditions in this adsorbent, it might be the next alternative natural adsorbent, where this natural adsorbent actualizes all factors to be effective in our lands.49-50
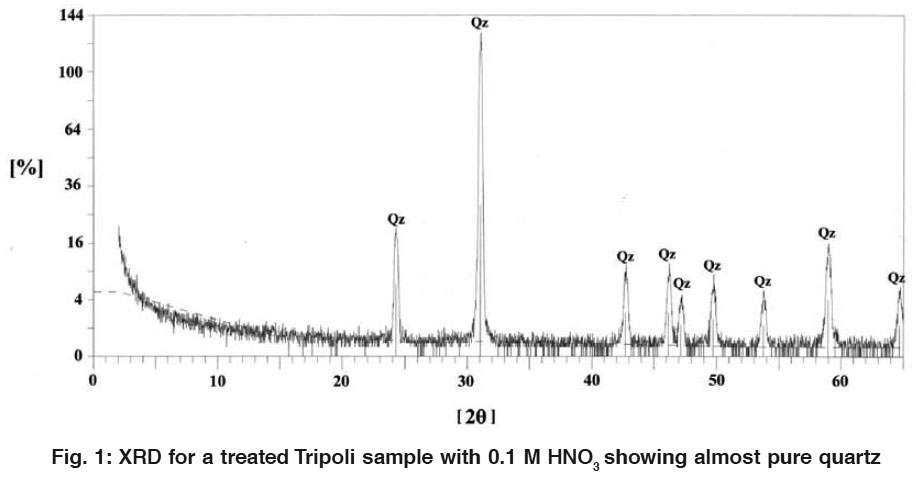 |
Figure 1: XRD for a treated Tripoli sample with 0.1 M HNO3 showing almost pure quartz Click here to view figure |
This work aims to investigate the utilization of Tripoli (MCCS) for removing heavy metals from the aqueous medium, which could be used as novel inorganic adsorbent material. This adsorbent can be used for the sorption of valuable and toxic Cu2+, Ni2+ and Zn2+ as single phase metal ions, which was accordant for the phosphate effluent water in our country. The equilibrium distribution of metal ion between the sorbent and the solution is important in determining the maximum sorbent capacity. Therefore, two isotherm models (Langmuir and Freundlich) will be used to assess the different isotherms and their ability to correlate the experimental data. The Langmuir equation will estimate the maximum adsorption capacity of complete monolayer coverage on the Tripoli surface. The Freundlich model is chosen to estimate the adsorption intensity of the sorbent towards the Tripoli.
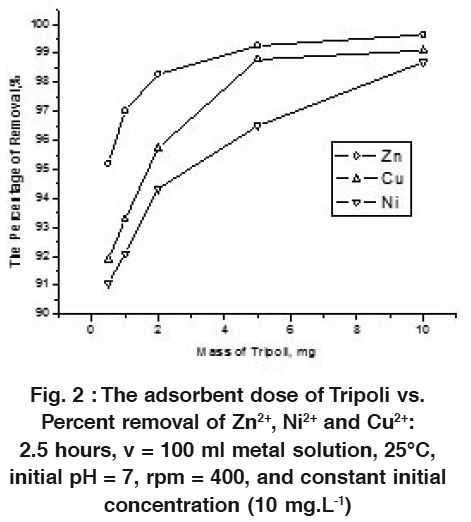 |
Figure 2 : The adsorbent dose of Tripoli vs. Percent removal of Zn2+, Ni2+ and Cu2+: 2.5 hours, v = 100 ml metal solution, 25°C, initial pH = 7, rpm = 400, and constant initial concentration (10 mg.L-1) Click here to view figure |
Experimental
Preparation of Tripoli
Tripoli was collected and the weathered surface was eliminated, cracked by a jaw crusher machine. In order to remove carbonate and other impurities, chemical treatment was performed by adding of 0.1 M HCl, 0.1 HNO3, and NH4Cl to the Tripoli fractions. After 24 hours, the solid phases were separated from the solution. Tripoli was pulverized by the ball mill and the mortar, and what was sifted out and set to -200 mesh (-74 mµ) was used and washed with excessive amounts of 1.0 M HCl through a suction filtration apparatus. Several drops of 1.00×10-3 M EDTA solution and 2 mL of NH3/NH4Cl buffer to an approximately 5 mL sample from the filtrate were added. Tripoli was then washed with excessive amounts of double deionized water until the filtrate gave a negative test for the chloride ion upon addition of several drops of 0.1 M AgNO3 solution to a sample from the filtrate. Ater the calicination, The Tripoli was stored in an oven at 110 °C.
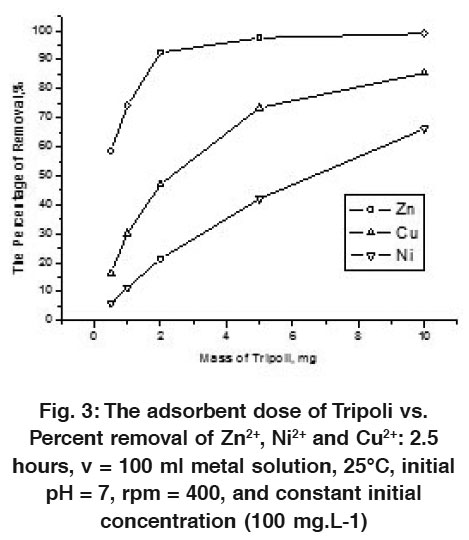 |
Figure 3: The adsorbent dose of Tripoli vs. Percent removal of Zn2+, Ni2+ and Cu2+: 2.5 hours, v = 100 ml metal solution, 25°C, initial pH = 7, rpm = 400, and constant initial concentration (100 mg.L-1) Click here to view figure |
The qualitative mineralogical analysis for the treated sample of Tripoli was determined using X-ray diffraction system (Seifert analyze model XRD 3003 TT). The powder samples were scanned between 0° and 65° 2ε, using Ni-filtered Cu Ká radiation, 40 kV/40mA, divergent and scattering slits of 0.02°mm, and receiving slit of 0.15 mm, with stepping of 0.01º and scanning speed of 3°/ min.
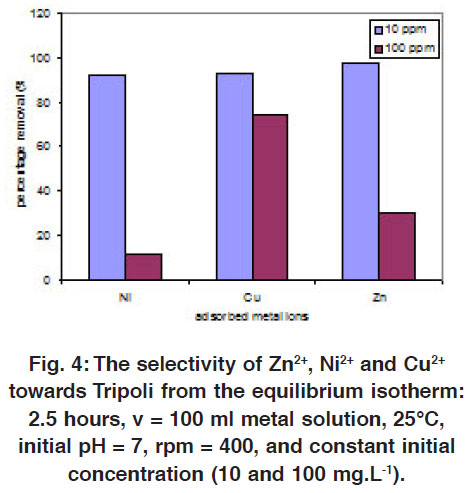 |
Figure 4: The selectivity of Zn2+, Ni2+ and Cu2+ towards Tripoli from the equilibrium isotherm: 2.5 hours, v = 100 ml metal solution, 25°C, initial pH = 7, rpm = 400, and constant initial concentration (10 and 100 mg.L-1). Click here to view figure |
Material
All chemicals used were analytical grade and were used as received. The metal salts of (Zn(NO3)2.3H2O, Cu(NO3)2.3H2O and Ni(NO3)2.3H2O) were purchased by commercial providers from Fluka Chemika. NaOH, HNO3 and HCl were purchased from Merck.
 |
Figure 5 a: The solubility effect of Zn2+, Cu2+, Ni2+ vs. pH values: pH = n, 4, 7 and 9.2 (where n = the original pH value for model solution before adsorption.), 2.5 hours, v = 100 ml metal solution, 25°C, rpm = 400, and constant initial concentration (10 mg metal ion × L-1) Click here to view figure |
Tripoli samples were collected from Aynon village (about 10 km in northwest from the campus of Mu’tah University, southern Jordan). The estimated reserve near the surface is about 6.4×106 million ton.49-50
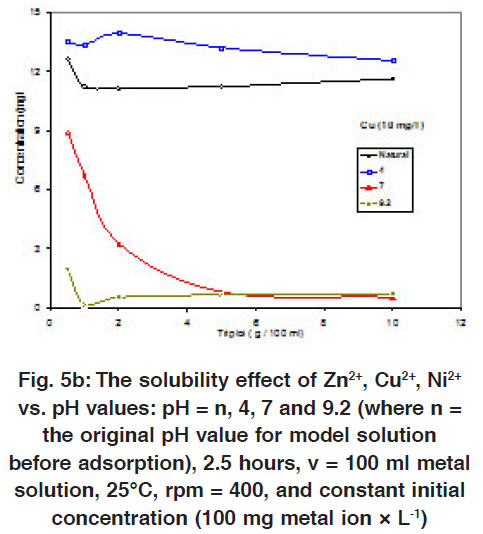 |
Figure 5 b: The solubility effect of Zn2+, Cu2+, Ni2+ vs. pH values: pH = n, 4, 7 and 9.2 (where n = the original pH value for model solution before adsorption), 2.5 hours, v = 100 ml metal solution, 25°C, rpm = 400, and constant initial concentration (100 mg metal ion × L-1) Click here to view figure |
Instruments
The concentration measurements of Cu2+, Ni2+ and Zn2+ were carried out by using a Perkin Elmer AAS Analyst 300, with graphite furnace HGA 800, and auto-sampler AS 72. The flame type was air-acetylene and the absorption wavelengths are 324.7 nm (for Cu2+) and 213.9 nm (for Zn2+). The pH was measured using hand held pH meters (315i/ SET). The shaking was carried out by using magnetic agitation in special flask. The adsorption flask was designed with special plastic form, where it includes path for the water circulation with inlet and outlet to control the temperature by water thermostat.
 |
Figure 5 c: The Effect of pH for the adsorption of Ni2+ by variant concentration of Tripoli: remaining concentration (mg.L-1) after the adsorption vs. Tripoli dosage g/100 ml model solution), pH = n, 4, 7 and 9 (where n = the original pH value for model solution before adsorption), 2.5 hours, v = 100 ml metal solution, 25°C, rpm = 400, and constant initial concentration (10 mg.L-1) Click here to view figure |
Methods
Stock solution (1000 mg L-1) of Cu2+, Ni2+ and Zn2+ were prepared by dissolving exact amount of metal salt (±0.01 g) in 1000 mL ultrapure deionized water (18 Ω cm). The standard model solutions of 10 and 100 mg L-1 were prepared by appropriate dilution. In order to keep pH constant, three kinds of buffers (pH = 4, pH = 7, and pH = 9.2) were used (Model Laboratory Rasayan) where one tablet was dissolved in 100 ml of deionized water.
 |
Figure 5 d: The Effect of pH for the adsorption of Cu2+ by variant concentration of Tripoli: remaining concentration (mg.L-1) after the adsorption vs. Tripoli dosage g/100 ml model solution), pH = n, 4, 7 and 9, (where n = the original pH value for model solution before adsorption), 2.5 hours, v = 100 ml metal solution, 25°C, rpm = 400, and constant initial concentration (10 mg.L-1) Click here to view figure |
The developed method from Ho (in 1995)51 was used in the batch adsorption tests, where three kinds of the heavy metal solutions (Cu2+, Ni2+ and Zn2+) are used. The test procedures are as follows:
- Several amounts from Tripoli were taken (0.5, 1, 2, 5, 1 and 10 mg) with 100 ml of metal ion solution and placed in the 250 ml of the plastic bottle flask (special design).
- Two different concentrations of each metal ions (100 and 10 mg.L-1) were considered.
- The plastic bottle flask were capped and shaken in a fixed magnetic agitation speed at 400 rpm for 2.5 hours.
- After complete the agitation time, the sample was filtered through Whatman 540 mm filter paper.
- The exact concentration of metal ions and filterable metal concentrations were determined by AAS without dilution (Perkin Elmer AAS Analyst 300, with graphite furnace HGA 800, and auto-sampler AS 72). The average standard deviation of the measurement was within 0.01 to 0.1 %.
- Qe was determined and Ce vs. Ce/Qe and lnCe vs. lnQe were plotted.
The results were expressed as the removal efficiency (% sorption) and adsorption capacity of the adsorbent:
The removal efficiency (% sorption) can be defined as
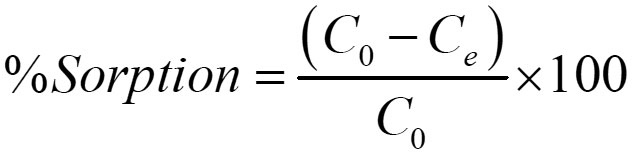
The adsorption capacity of Tripoli (mg metal/g Tripoli) was calculated by the following equation:
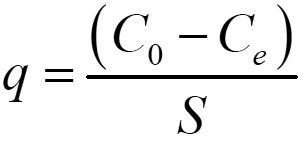
Where
q : metal ions adsorbed by Tripoli (mg/g)
C0 : initial metal concentration (mg/L)
Ce : metal concentration at equilibrium (mg/L)
S: dosage= m/V
V : volume of metal solution (L)
m : mass of Tripoli (g)
Results and Discussion
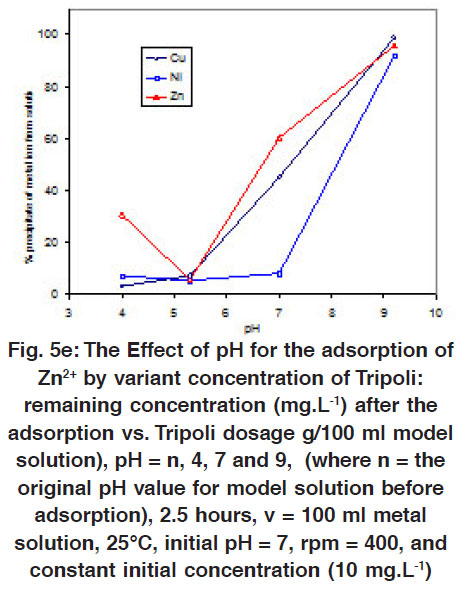 |
Figure 5 e: The Effect of pH for the adsorption of Zn2+ by variant concentration of Tripoli: remaining concentration (mg.L-1) after the adsorption vs. Tripoli dosage g/100 ml model solution), pH = n, 4, 7 and 9, (where n = the original pH value for model solution before adsorption), 2.5 hours, v = 100 ml metal solution, 25°C, initial pH = 7, rpm = 400, and constant initial concentration (10 mg.L-1) Click here to view figure |
Adsorption and Analysis of Natural Tripoli
Tripoli is considers a common name for the natural rocky crystalline silica that has sub-micrometer crystals (which is formed from amorphous, often biogenic). This silica undergoes the compacting over geologic time and then produces the Microcryptocrystalline Silica (MCCS). The physico-chimical properties of Tripoli were published.52 This kind of silica has so fine pores that are saturated with water, unless they are dried. Also, these pores have a certain cavities with different sizes for adsorbing the suitable metal ions. On the surface, it is ending with Si-O-H, which might bind metal ions via the coordination aspects. Therefore, the heavy metals can be stabilized by these pores and then the toxicity is somewhat removed. This is due to in situ encapsulated of the metal ions inside silica cavities and pores. In this case, the metal ions remain inert in the soil and environment. Basing on these fundamental aspects, the adsorption process via Tripoli is considering the potential alternative for removing heavy metals especially for these countries of limiting resources. This is due to the several reasons, for example, it has easy handling, low cost, naturally occurring, huge quantity, and safe for the environment. In the same time, the results reveal that all metal ions exhibit strong affinity for adsorbing heavy metals on Tripoli.
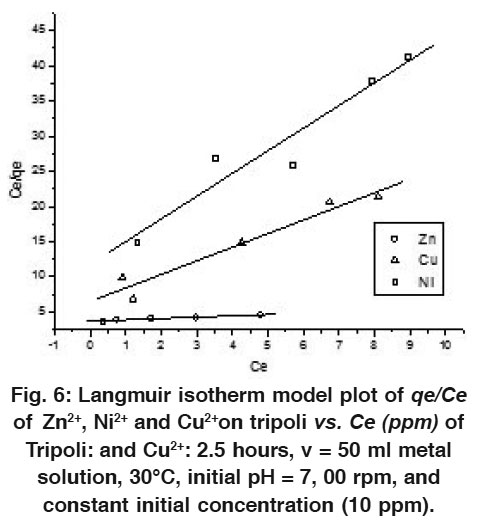 |
Figure 6: Langmuir isotherm model plot of qe/Ce of Zn2+, Ni2+ and Cu2+ on tripoli vs. Ce (ppm) of Tripoli: and Cu2+: 2.5 hours, v = 50 ml metal solution, 30°C, initial pH = 7, 00 rpm, and constant initial concentration (10 ppm). Click here to view figure |
Diffractograms of the treated samples (Fig. 1) showed only quartz as main mineral phase by the percentage 100% as a microcryptocrystalline (MCCS) and other forms of silica. Whereas, the montmorillonite and dolomite were destroyed. The characteristic calcite peak at 34.2 (2ε) was reduced completely. This indicates the complete removing of the impurities through the chemical treatments by acids and bases. Calcinations did not loss of the original silica. It was used due to the decomposition of last concentrations of carbonates and organic matter as well as dehydration of the structural and adsorbed water.
The geochemical data have been shown the contents of non-treated Tripoli in the percentage of greater than 92.45 wt % of MCCS (SiO2). The specific gravity of Tripoli was found in the range of 2.4-2.6, with white creamy color.50,52 Table 1 presents the main chemical composition of the natural Tripoli. The determination description of these parameters can be found in.53
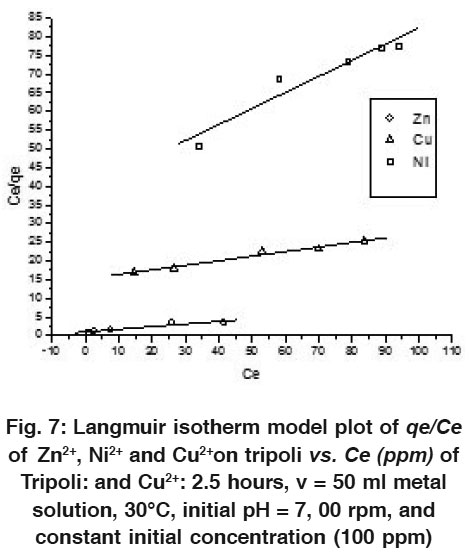 |
Figure 7: Langmuir Isotherm Model Plot of qe/Ce of Zn2+, Ni2+ and Cu2+ on Tripoli vs. Ce (ppm) of Tripoli: and Cu2+: 2.5 hours, v = 50 ml Metal Solution, 30°C, Initial pH = 7, 00 rpm, and Constant Initial Concentration (100 ppm) Click here to view figure |
The removing of original metals inside the natural Tripoli can be tested using EDTA. The test was performed by adding several drops of 1.00 x 10-3 M EDTA solution and 2 mL of NH3/NH4Cl buffer to an approximately 5 mL sample from the filtrate, the negative test for the presence of metal ions is indicated by appearing the blue color of EDTA. Tripoli was then washed with excessive amounts of double distilled water until the filtrate gave a negative test for chloride ion upon addition of several drops of 0.1 M AgNO3 solution to a sample from the filtrate.
Effect of Tripoli Dosage
The effect of Tripoli dosage is discussed by using different amounts of adsorbent (viz. 0.5, 1.0, 2.0, 5.0, and 10 mg), while the other parameters (T = 25°C. initial metal concentration = 10 or 100 mg.L-1, pH = 7, rpm = 400, and the contact time = 2.5 hours) are keeping constant (see Figs. 2 & 3). The results reveal the strong adsorbing affinity for all metal ions via Tripoli at around pH = 7. These adsorbed metal ions reach the maximum percentage at about 100 mg Tripoli × L-1 (see Figs. 2 and 3). This adsorbed percentage increases by increasing Tripoli dosage. Figure 2 clarifies the adsorption of metal ions in the diluted level (10 mg metal ion × L-1) vs. adsorbent dosage, where attain the maximum percentage at about 95-99 % for all adsorbed metal ions. Fig. 3 exhibits the low adsorption percentage of the metal ions in the high level concentration (100 mg metal ion × L-1), this is just when compares it with the diluted level. In this case, the percentages have been calculated at about 60, 85, and 90 % for Zn2+, Cu2+ and Ni2+, respectively.
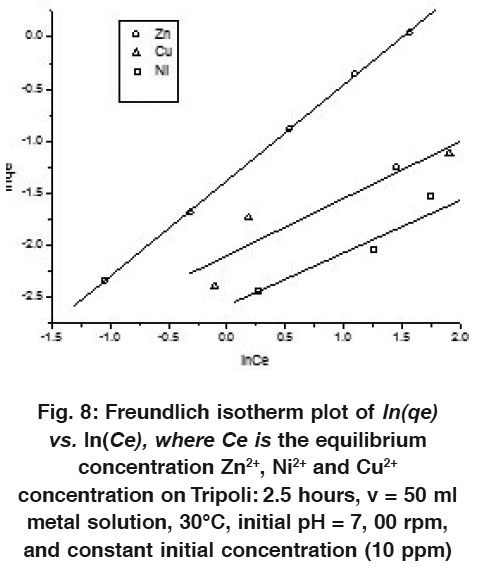 |
Figure 8: Freundlich Isotherm plot of ln(qe) vs. ln(Ce), where Ce is the Equilibrium Concentration Zn2+, Ni2+ and Cu2+ Concentration on Tripoli: 2.5 hours, v = 50 ml Metal Solution, 30°C, Initial pH = 7, 00 rpm, and Constant Initial Concentration (10 ppm) Click here to view figure |
The adsorption percentages of Zn2+ and Cu2+ ions increase sharply by increasing adsorbent doses in the range of 0.5 g to 5 g (approx.) and then it slightly increases in the range of 5 g to 10 g of Tripoli, while the percentage removal of Ni2+ increases sharply in the whole range of 0.5 to 10 g of Tripoli. In this level, the maximum adsorption might be attained. This means, the amount of ions bounded to the adsorbent are in the maximum numbers, while the amount of the free ions remains constant for any further addition from adsorbent. Generally, the removal percentages increase by increasing the Tripoli dosage for both diluted and concentrated levels. This is due to the greater availability of the surface area.
Variation of Initial Metal Ion Concentration
The effect of the Cu2+, Ni2+ and Zn2+ initial concentration of vs. the adsorption percentages on Tripoli is studied by using both diluted and concentrated solutions (10 to 100 mg × L-1), while keeping all other parameters constant (T = 25°C, pH = 7, rpm = 400, and the contact time = 2.5 hours). As clearly appear in the Fig. 4, the removal percentages increase by increasing the initial metal concentrations (10 vs. 100 mg metal ion × L-1). Such observations are quite common,52, 54 while it is in opposite of using natural zeolite.20 This behavior is connected with the diffusion process of the metal ions through the micro-channel and pores on the silica, where the metal ions are in competitive to diffuse through these micro-pores. This competitive will lock the inlet of channel on the surface, in the same time it prevents the metal ions from passing deeply inside the silica, i.e. the adsorption occurs on surface only. Therefore, the removal percentages increases for each case of the diluted metal ions (10 mg × L-1). While the adsorption is concentration independent at the level of the concentrated metal ions, (above 100 mg × L-1). This may return to the reason of metal ions ratio to the available adsorption sites are modicum. That means the competition for the adsorption sites become fierce at high level concentrations.
 |
Figure 9: Freundlich Isotherm Plot of ln(qe) vs. ln(Ce), where Ce is the Equilibrium Concentration Zn2+, Ni2+ and Cu2+ Concentration on Tripoli: 2.5 hours, v = 50 ml Metal Solution, 30°C, initial pH = 7, 00 rpm, and Constant Initial Concentration (100 ppm). Click here to view figure |
In general, the removal percentages are high (Approx. above 92%), where the diluted Zn2+ is characterized as the highest removal efficiency (Figure 4). The order of the percentage removal via Tripoli is found as Zn2+ > Cu2+ > Ni2+. In our suggestion, the passing path of Ni2+ interrupts on the Tripoli surface through the adsorption process more than Cu2+ and then Cu2+ > Zn2+. This behavior relates to the metal-silica coordination aspect. It is known that the silica has Si-O-Si backbone. The oxygen in the silica backbone has two lone pairs, and they are available to interact with the empty or partially empty d-orbital of the metal ion.
The electronic states of the Zn2+, Cu2+ and Ni2+ are d10, d9 and d8, respectively. Zn2+ has full orbital and diffuses easily inside Tripoli structure without any strong interruption. The passing path of Ni2+ slightly interrupts through the interaction of Ni2+ with oxygen atoms on the silica surface, i.e. locking the surface pores. This interaction may prevent other ions to move inside the pores and then increase the removal percentage. Therefore, it is expected that Ni2+ will be adsorbed on the surface and near the surface more than Zn2+. The copper has the moderate behavior on the surface between Zn2+ and Ni2+.
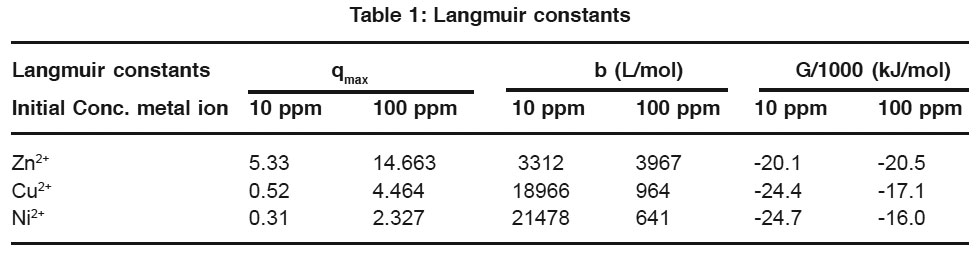 |
Table 1: Langmuir Constants Click here to view table |
This trend was compared with treated clay (Sh2 adsorbent).55 The order of the removal capacity in the batch adsorption via Sh2 adsorbent has recently reported as Cu2+>Ni2+>Zn2+ for both natural and treated clay adsorbent. In our obtained trend is found easier for the explanation more than the reported study. This is due to the chemical composition of Sh2 clay where contains several adsorbent components, and then the adsorption is carrying out on several adsorbents, for example, silica, 34% calcite and chlorite, while Tripoli has one pure quartz component. In addition, the adsorption for these metals on Sh2 has a complicated story, where depends on the techniques and conditions. For example, the uptake capacity of metals in column experiments was reported as Ni2+>Zn2+>Cu2+, and differed from that established in the batch uptake.55
Effect of pH
Figure 5(a-b) shows the effect of pH vs. the sorbet (Zn2+, Cu2+, Ni2+) behavior in the model solutions before adsorbing. The model solution (10 and 100 mg.L-1) was prepared in the deionised water, and their pH values were changed by the buffer solutions. After forming and filtrating supernatant, the metal ion concentrations were detected by the AAS and then the remaining concentrations were calculated. Figure 5a shows the effect pH on metal ions in case of diluted model solution (10 mg.L-1). This figure clarifies the precipitating of each metal ion in the basic medium (pH greater than 7), which were found about 70% for the Ni2+ and 100% for both metals of Zn2+ and Cu2+. It was found so complicated behaviors for the same metals in the concentrated model solutions (100 mg.L-1) in both acidic and basic medium, which is clearly shown in the Figure 5b. Basing on these results, this study denoted the adsorption on Tripoli in the neutral medium.
 |
Table 2: Freundlich constants Click here to view table |
The values of pH are considering one of the important parameters for the adsorption metal ions on Tripoli. The initial pH value for the solutions were varied, which was started from the original value for the model solution (pH = n) to the pH = 4, 7 and 9.2. In this group of experiments, the amount of adsorbent (0.5, 1, 2, 5 and 10 mg of Tripoli/100 ml of model solution), the concentration of adsorbate (10 or 100 mg.L-1), contact time (2.5 hours), Temperature (25 ºC) and the agitation speed (400 rpm) are kept constant. Figures 5(c-e) show the removal of heavy metals (Cu2+, Ni2+, Zn2+) on Tripoli where completes at about pH = 7 for all metals. Around this value, the percentage removal increases by increasing Tripoli dosage. The adsorption actualizes the maximum percentage for the Zn2+, Cu2+ and Ni2+ at 10 and 5 mg Tripoli/ 100 ml model solution, respectively.
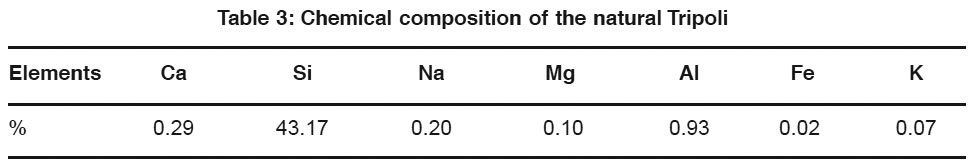 |
Table 3: Chemical composition of the natural Tripoli Click here to view table |
The metal ions were not adsorbed in acidic medium (0 %) as well as they were precipitated in basic medium (at about 95%). The adsorption can not be carried out in the basic medium due to the precipitating metal ions (sorbet: Zn2+, Cu2+ and Ni2+) as metal hydroxides M(OH)2 (see Figs. 5a- 5b). Furthermore, the silica backbone structure (sorbent: Tripoli) hydrolyzes by increasing the number of protons in the acidic medium. Therefore, the best pH value must be at about 7 to actualize and achieve the maximum removal, which are clearly explicit in the Figures 5(c-e). For this reason, there are no removal incase of acidic medium as appeared in theses figures. These results regarding the pH effect are matching with the reported data when applied in wastewater. Where the extent of adsorption for these metals was largely independent of pH over a wide pH range, but was significantly reduced at very low (pH< 4) and to a lesser extent at high pH (pH> 10)56 Therefore, good adsorption efficiency via Tripoli is expected at intermediate pH values likely to be encountered with many waste waters.
 |
Table 4: Adsorption potential of various sorbents Click here to view table |
Isotherm Models
To quantify adsorption capacity of Tripoli for the removal of Zn2+, Cu2+ and Ni2+ from the solution model of wastewater, the data were fitted into the Langmuir and Freundlich adsorption isotherm models.54-57 The Langmuir model supposes a monolayer sorption with a homogeneous distribution of sorption sites and sorption energies, without interactions between the sorbet molecules. It can be represented in a linear form as follows

Whereqe is the equilibrium amount of metal ions sorbet per unit mass of Tripoli (mg/g), qmax is the maximum metal uptake per unit mass of Tripoli (mg/g), Ce is the equilibrium concentration of metal ions in solution (mg/dm3) and b is Langmuir constant (dm3/mol). b is related to energy of sorption which reflects quantitatively the affinity between the Tripoli and metal ions.
Therefore, a plot of C e  q e versusCe , gives a straight line of slope 1
q e versusCe , gives a straight line of slope 1 q max and intercept1
q max and intercept1 ( q max b ) as shown in Figs (6 and 7) for 10 and 100 ppm initial solution, respectively for each metal ions of Cu, Zn, and Ni.
( q max b ) as shown in Figs (6 and 7) for 10 and 100 ppm initial solution, respectively for each metal ions of Cu, Zn, and Ni.
The very useful relationship between standard free energy change, G 0 , and the Langmuir constant, b, is given by the following equation.43, 57
G 0 = RT ln b
where R is universal gas constant (8.314 J/mol K), and T is the absolute temperature in K. The values of standard free energy change are listed in the Tables 2 and 3 for the three heavy metals at 10 ppm and 100 ppm. The negative value of G0 confirms the feasibility of the process and the spontaneous nature of adsorption with a high preference for metal ions (Zn2+, Cu2+ and Ni2+, respectively) to adsorb onto Tripoli.
In contrast to the Langmuir model, the Freundlich isotherm is more widely used but it provides no information on the monolayer adsorption capacity. The linear form of this model is given as follow:
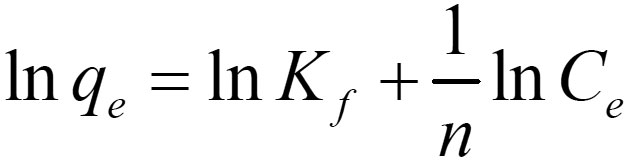
Where K f and n are Freundlich constants, indicating the adsorption capacity and the adsorption intensity respectively. Theses values of and n are, respectively, determined from the intercept and slope of plotting versus.
The linear form of Freundlich adsorption isotherms of each metal ion (Zn2+, Cu2+ and Ni2+) at 10 ppm and 100 ppm are shown in the Figs. 8 and 9.
The goodness fit of the experimental data is measured by the regression coefficients, R2 as shown in the Tables 2 and 3. These values indicate to the applicability of the Langmiur model to Ni+2 and the applicability of Freundlich to Zn2+ and Cu2+ ions.
The Langmuir constant qmax (maximum adsorption capacity) and the Freundlich constant Kf were obtained from the linear equations. The values are summarized in the Tables 2 and 3.
It can be observed that the maximum adsorption capacity of Tripoli is the highest for Zn2+ followed by Cu2+ and Ni2+ for both initial concentrations 10 ppm and 100 ppm. The Freundlich constant Kf for Zn2+ is greater than the other heavy metals for initial concentration of 10 ppm and 100 ppm. The values of n for all three heavy metals were obtained as greater than one, which indicates good adsorption of these metals onto Tripoli.
Table 4 summarized the various adsorbent that used for adsorbing of Zn2+, Cu2+ and Ni2+. The comparison has been made in terms of (mg/g). Although a direct comparison of Tripoli with other reported adsorbents is difficult due to varying experimental conditions employed in those studies, but in general the adsorption capacity of Tripoli is considerably higher than many of those adsorbents.
Conclusion
Tripoli can be used for the removal of valuable and toxic Cu2+, Ni2+ and Zn2+ as single phase metal ions from the aqueous medium, which could be used as novel inorganic adsorbent material in the poor countries. This is due to the several reasons, for example, it has easy handling, low cost, naturally occurring, huge quantity, and safe for the environment. In the same time, the results reveal that all metal ions exhibit strong affinity for adsorbing via Tripoli, where attain the maximum percentage at about 95-99 % for all adsorbed metal ions. The suitable adsorption conditions to actualize the maximum sorption must be at about pH = 7, at about 50 to 100 mg Tripoli ×L-1 and using the diluted solution. The calculated values by Langmuir and Freundlich models confirms the experimental data, which has the spontaneous nature as Zn2+>Cu2+>Ni2+. This is in consistence with Langmuir and Freundlich models that gives the same order. The equilibrium adsorption could be described by Langmuir for Ni+2 and by Freundlich adsorption isotherms for Zn+2 and Cu+2 ions. The Tripoli adsorption capacities of Zn+2, Cu+2 and Ni+2 ions are 14.66, 4.46 and 2.33 mg g”1 respectively. This study is based on a simple component with single metal ions; the competition among ions should be including in future studies in order to simulate waters in the environment.
Acknowledgements
The XRD analysis was done at the Physics Department/ Mutah University (Jordan). The authors wish to thank both chemists of Samah Ramadneh and Nancy Okkeh (Department of chemical Science/ Mutah University) for their experimental contributions.
References
- Duffus, J.H. ´´Heavy metals“-A meaningless term, IUPAC technical report, Pure Appl. Chem. (2002) 74: 5,793.
- Jiries, A., El-Hassan, T., Al-Hweiti, M. and Seiler, K-P. Evaluation of the effluent water quality produced at phosphate mines in central Jordan, Mine Water and environment. (2004) 23: 133.
- Smyth, D.J., Blowes, D.W., Ptacek, C.J., Baker, M.J., Ford, G., Foss, S., Bernstene, E. Removal of phosphate and water boron pathogeneses from wastewater effluent using Permeable reactive materials, ground and water: theory to practice, Proceeding of the 5th Canadian geotechnical and 3ed joint IAH-CNC and CGS ground water speciality conference, Niagara falls, Ontario, (2002) Oct.20-23: 1123 –1128.
- Vandenhove, H. European sites contaminated by residues from the ore-extracting and –processing industries. International Congress Series. (2002) 1225: 307.
- National Research Council (NRC). Drinking water and health National Academy of Sciences, Washington DC (1977).
- Inglezakis, V.J., Loizdou, M.D. and Grigoropoulou, H.P. Ion exchange of Pb2+, Cu2+, Fe3+, and Cr3+ on natural clinoptilolite: selectivity determination and influence of acidity on metal uptake J. Colloid Interface Sci. (2003) 261: 49.
- Harte, J., Holdren, C., Schneider, R., and Shirley, C. Toxics A to Z. A guide to everyday pollution hazards. University of California Press, Berkeley CA: (1991) 103-105.
- Gardea-Torresdy, J.L., Ppolette, L., Aarteaga, S., Tiemann, K.J., Bibb, J., and Gonzalez, J.H., Determination of the Content of Hazardous Heavy Metals on Larrea Tridentata Grown Around a Contaminated Area. Proceedings of the Eleventh Annual EPA Conf. on Hazardous Waste Research, (HSRC/WERC Joint Conference on the Environment), Edited by L.R. Erickson, D.L. Tillison, S.C. Grant, and J.P. McDonald, Albuquerque, NM, (1996) 660-669.
- Meagher, R.B. Phytoremediation of toxic elemental and organic pollutants. C.Op. in Plant Biol. (2000) 3: 153.
- Nanda, P.B.A., Dushenkov, V., Motto, H., and Raskin, I.. Phytoextraction: the use of plants to remove heavy metals from soil. Environ. Sci. Technol. (1995) 29: 1232.
- Blaylock, M.J. and Huang, J.W. Phytoextraction of metals, In: I. Raskin and Ensley BD (Ed.): Phytoremediation of toxic metals: using plants to clean up the environment, John Wiley and Sons, Inc. Toronto, Canada, (2000) 303.
- Oouki, S.K. and Kavnnagh, M. Water Sci, Technol. (1999) 39: 115.
- Malliou, E., Loizidou, M. and Spyrellis, N. Sci, Total Environ. (1994) 149: 139.
- Zamnow, M.I., Ecichbaum, B., Sangren, K. and Shanka, D. Sep. Sci. Technol. (1990) 25(13-15): 1555.
- Park, J.-B., Lee, S.-H, Lee, J.-W. and Lee, C.-Y. Lab scale experiments for permeable reactive barriers against contaminated groundwater with ammonium and heavy metals using clinoptilolite (01-29B) J. Hazard. Mater. (2002) 95(1/2): 65-79.
- Babel, S., Kurniaan, T.A. Low-cost adsorbents for heavy metals uptake from contaminated water: a review. J. Hazard Mater. (2003) 97: 219.
- Curkkovic, L., Cerjan-Stefanovic, S., Fillipan, T. Metal ion exchange by natural and modified zeolites. Water Res. (1997) 31(6): 1379.
- Panayotova, M. and Velikov, B. Kinetics of heavy metal ions removal by use of natural zeolite. J. Environ. Sci. Health A. (2002) 37(2): 139.
- Inglezakis, V.J. and Grigoropouou, H.P. Applicability of Simplified Models for the Estimation of Ion Exchange Diffusion Coefficients in Zeolites. J. Colloid Interface Sci. (2001) 234: 434.
- Erdem, E., Karapinar, N., and Donat, R. The Removal of Heavy Metal Cations by Natural. Zeolites. J. Colloid Interface Sci. (2004) 280: 309.
- Doula, M. K., and Ioannou, A. The effect of electrolyte anion on Cu adsorption– desorption by clinoptilolite. Micropor. Mesopor. Mater. (2003) 58: 115.
- Vasylechko, V.O., Gryshchouk, G.V., Kuz´ma, Yu.B., Zakordonskiy, V.P., Vasylechko, L. O., Lebedynets, L. O. and Kalytovs´ka, M.B. Adsorption of cadmium on acid-modified Transcarpathian clinoptilolite. Micropor. Mesopor. Mater. (2003) 60: 183.
- Geberemedhin-Haile, T., Olguin, M. and Solache-Rios, T., M. Water Air Soil Pollut. (2003) 148: 179.
- Appegate L.E. Membrane separation processes. Chem. Eng. (1984) 91: 64.
- Sengupta, A. K. and Clifford, D. Important process variables in chromate ion exchange. Environ. Sci. Technol. (1986) 20: 149.
- Geselbarcht, J. Micro Filtration/Reverse Osmosis Pilot Trials for Livermore, California, Advanced Water Reclamation, Water Reuse Conference Proceedings, AWWA, (1996) 187.
- Schnoor, J.L. Phytoremediation, TE-97-01, Ground Water Remediation Technologies Analysis Centre, Pittsburgh, PA. (1997).
- Choo, P.L., Siefet, K.S., and Sparapany J.W. Novel Chemical Solutions to Heavy Metal Removal from Wastewater. Paper presented at WPCF 65th Annual Conference, New Orleans, Louisiana, (1992) pp. #133-139:
- Groffman, A., Peterson S. and Brooking D. Removing Lead from Wastewater Using Zeolite. Water Environment & Technology, (1992) 54-59
- Capaccio, R.S. Metals Control. Industrial Wastewater,(1996) 21-24.
- Geseelbarcht J. Micro filtration/ Reveres osmosis pilot trials for Livermore, California, Advanced Water Reclamation, in: Water Reuse conference proceedings, AWWA,(1996) 187.
- Bailey, S.E., Olion, T.J., Bricka, R.M., Adrian, D.D. A review of potentially low-cost sorbents for heavy metals. Water Res (1999). 33(11): 2469.
- Banat, F., AL-Asheh, S. and Mohai, F. Multi-metal sorption by spent Animal Bones. Sep. Sci. and Technol. (2002) 37(2): 311.
- AL-Asheh, S., Banat, F., Al-Omari, R. and Dunvjak, Z., Predictions of binary sorption isotherms for the sorption of heavy metals by pine bark using single isotherm data. Chemospher. (2000) 41:5 (2002) 659.
- Randall, J.M., Hautala, E., and Waiss, A.C. Binding of heavy metal ions by formaldehyde polarized peanut skin, J. Appl. Polym. Sci. (1978) 22: 379.
- Tee, T.W. and Khan, R.M. Removal of lead, cadmium and zinc by tea waste leaves. Environ. Technol. Lett. (1988) 9: 1223.
- AL-Asheh, S. and Dunvjak, Z. Sorption of heavy metals from synthetic metal solutions and industrial wastewater using plant materials. Water Quality research Journal of Canada. (1999) 43(3): 481.
- Roy, D., Greenlaw, P.N. and Shane, B.S. Adsorption of heavy metals by green algae and ground rice hull. J. Environment. Sci. Health. (1993) A28(1): 37.
- a) AL-Asheh, S., and Banat, F., Adsorption of copper and zinc by oil shale. Environ. Geol. (2001) 40(6): 693. b) AL-Asheh, S., and Banat, F., Adsorption of zinc and copper ions by the solid waste of the olive oil industry. Adsorption Science and Technology. (2001) 19: 2,117.
- Vithanage, M., Chandrajith, R., Bandara, A., and Weerasooriya, R. Mechanistic modeling of arsenic retention on natural red earth in simulated environmental systems. Journal of Colloid and Interface Science. (2006) 294 :265.
- Kapoor, A. and Viraraghavan, T.F., Cullimore, D. R. Removal of heavy metalsusing fungus Aspergillus niger. Biosours. Technol. (1999) 70: 1, 95.
- Chiron, N., Guilet, R. and Deydier, E. Adsorption of Cu(II) and Pb(II) onto a grafted silica: Isotherms and kinetics models. Water Res. (2003) 37(13): 3079.
- Singh, S., Rai, B.N. and Rai, L.C. Ni(II) and Cr(VI) sorption kinetics by microcystis in single and multimetallic system. Process Biochem. (2001) 36(12): 1205.
- Reddad, Z., Ge´rente, C., Anndres, Y. and Cloirec, P. Le. Adsorption of several metal ions onto a low-cost biosorbent: kinetics and equilibrium studies. Environ. Sci. Technol. (2002)36(9): 2067.
- Ho, Y.S. and Mckey, G., The kinetics of sorption of divalent metal ions onto sphagnum moss peat, Water Res. (2002) 34(3): 735.
- Al-Anber, M. and Al-Anber, Z. Utilization of natural zeolite as ion-exchange and sorbent material in the removal of iron. Desalination. (2008) 225: 70-81.
- Al-Anber, M. Removal a Model Solution of Trivalent Iron Using Jordanian Natural Zeolites. Asian J. Chem. (2007) 19(5): 3493-3501
- Al-Anber, Z. A. and Matouq, M. Batch adsorption of cadmium ions from aqueous solution by means of olive cake. J. of Hazard. Mater. in press, Available online 31 May 2007 (2008).
- Bender F. Geology of Jordan. Contribution to the regional geology of the world. (Berlin: Gebrueder Borntraeger),(1974) 174.
- Abed, A. Geology of Jordan. Al-Nahda Al-Islamiah Library. P 232. (Arabic) (1982):
- Ho, Y.SAdsorption of Heavy Metals from Waste Streams by Natural Materials, PhD Thesis, the University of Birmingham, UK. (1995).
- Khoury, H. Industrial rocks and minerals in Jordan, (occurrences, properties, and ortigin). Univ. of Jordan Publications. Amman. P240 (In Arabic)) (1982).
- Abu-Ajamieh, M., Mineral resources of Jordan: Amman, Jordan, Ministry of Energy and Natural Resources,(1989) 147.
- Hasar, H. Adsorption of nickel(II) from aqueous solution onto activated carbon prepared from almond husk. J. Hazard. Mater. (2003) 9: 49.
- Vengris T., Binkiene R., Sveikauskaite A. Nickel, copper and zinc removal from waste water by a modified clay sorbent. Applied Clay Science (2001) 18: 183–190.
- Djati-Utomo D. H. and Hunter K. A. Adsorption of heavy metals by exhausted coffee grounds as a potential treatment method for waste waters. e-J. Surf. Sci. Nanotech. (2006) 4: 504-506.
- Casey, T.J. Unit Treatment Processes in Water and Wastewater Engineering, John Wiley and Sons Ltd, England, (1997) 113-114.






