Heavy metal adsorption by clinoptilolite from aqueous solutions
P.P. Wani * and S.R. Thorat
1
School of Environmental and Earth Sciences North Maharashtra University,
Jalgaon,
Maharashtra
India
DOI: http://dx.doi.org/10.12944/CWE.3.1.20
The present investigation has been carried out to assess a chromium, cobalt, and cadmium from wastewaters by natural and modified zeolites were examined by using batch type method. A clinoptilolite type synthetic / natural zeolite were pretreated with HCl and HNO3 to improve the adsorption capacity for heavy metals. The removal efficiencies and kinetics of heavy metals such as chromium, cobalt and cadmium on natural modified zeolites were determined. The kinetics of adsorption indicates the process to be diffusion controlled. This process is very well work in industrial treatment plant of various industries using heavy metals for their production.
Copy the following to cite this article:
Wani P.P, Thorat S.R. Heavy metal adsorption by clinoptilolite from aqueous solutions. Curr World Environ 2008;3(1):135-141 DOI:http://dx.doi.org/10.12944/CWE.3.1.20
Copy the following to cite this URL:
Wani P.P, Thorat S.R. Heavy metal adsorption by clinoptilolite from aqueous solutions. Curr World Environ 2008;3(1):135-141. Available from: http://www.cwejournal.org/?p=785
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2008-02-24 |
|---|---|
| Accepted: | 2008-03-12 |
Introduction
Heavy metals are common pollutants found in various industrial effluents. The stricter environment regulation on the discharge of heavy metal it is necessary to develop various technologies for removal. Waste streams containing low to medium level of heavy metals are often encountered in metal plating facilities, electroplating, mining operation, fertilizer, battery, manufacture, dyestuffs, chemical pharmaceutical, electronic device manufactures and many others. Most of heavy metals are highly toxic and are not biodegradable; therefore they must be removed from the polluted streams in order to meet increasingly stringent environmental quality standards. Many methods including chemical precipitations, electro deposition, ion exchange, membrane separation and adsorption have been used to treat such streams of these methods, traditional chemical precipitation is the most economic but is inefficient for dilute solutions. Ion exchange and reverse osmosis are generally effective, but have rather high maintenance and operation costs and subject to fouling. Adsorption is one of the few promising alternatives for this purpose, especially using low cost natural sorbets such as agricultural wastes, clay materials, zeolite, biomass and seafood processing wastes (Kesraoui-Ouki S et.al. 1994, Orhan Y. et.al.1993).
Clinoplolite is a mineral zeolite of the Heulandite group. The structures of zeolites consists of a three dimensional framework of SiO4 and AlO4 tetrahedral (Curkovic L. et.al. 1997). The aluminum ion is small enough to occupy the position in the center of tetrahedron of four oxygen atoms, and isomorphous replacement of Al3+ for Si4+ raises a negative charge in the lattice. The net negative is balanced by the exchangeable cation (sodium, potassium and calcium). These cations are exchangeable with certain cations in solution such as lead, cobalt, zinc and manganese. The fact that zeolite exchangeable ions are relatively innocuous (sodium calcium and potassium ions) makes them particularly suitable for removing undesirable heavy metal ions from industrial effluent waters. One of the earliest applications of a natural zeolite was in removal and purification of cesium and strontium radioisotopes. Heavy metal for Clinoptilolite and radionuclide ions adsorption has investigated by many investigators (Curkovic L. et.al. 1997, Akyuz T. et.al. 2000, Wani et.al., 2007).
The removal of heavy metal ions from industrial wastewaters using different adsorbents is currently of great interest. Activated carbon has been tested for the removal of inorganic ions from aqueous solution. However, in order to minimize processing cost for these effluents, recent investigations have been focused on the use of low cost adsorbents. Clinoptilolite was shown to have high selectivity for certain heavy metal ions such as Pb2+, Cd2+, Zn+ and Cu+. A significant number of researchers have done experiments, which have determined different selectivity sequences of natural zeolites for range of various metals, but they have all agreed that Clinoptilolite shows a strong affinity for lead and cobalt. Most of them have suggested that pretreatment of natural zeolites enhances their ion-exchange ability (Malliou E. et.al. 1994, Kim K.S., Choi H.C. 1998 and Wani and Thorat, 2007). The reaction and diffusion control models have been proposed to describe the adsorption kinetics, which are based on the relative importance of the chemical reaction to diffusion transfer. The results of the kinetic studies by Eligwe et. al. showed that the adsorption reaction is first order with respect to the metal cation solution concentration. It was found that the rate constant was a function of metal ion concentration, PH, and initial concentration. The uptake kinetics of cobalt and selenite was studied by Papelis et.al. The rate data were interpreted with a diffusion model, in which a linear isotherm was employed to express the local equilibrium relationship. The above models may be satisfactory under the particular experimental conditions; however, these models and their parameters normally are system specific (PH-dependent) and cannot be applied to other conditions (Yiacoumi S. and Tien C. 1995, Wani and Thorat, 2007).
The aim of the investigation is to study the adsorption mechanism of Co (II), Pb (II) and Cr (VI) ions onto natural and modified zeolites from wastewater.
Material and Methods
Natural zeolites sample was obtained from Biga-Canakkale region of Turkey. It was ground to approximately 200-mesh size powder. A given amount of the material was washed with deionised water three-four times to remove any dust and other water-soluble impurities. The sample was then dried in an electric oven at 150-200°C for 2-3 hrs before using for adsorption purpose.
 |
Table 1: Chemical composition of zeolite sample tested (%) Click here to view table |
Metal removal studies were carried out using Clinoptilolite in three different forms; one untreated and two treated samples. Sample 1; natural zeolites, Sample 2; natural zeolite was treated with 2M HCl solution over period of 24 hrs, after washing, modified zeolites were dried at 105°C for 1 hr. sample 3; the zeolite was prepared with 2M HNO3 solution over a period of 24 hr.
The chemical composition of the natural sample was determined by XRF analysis. The analysis was performed on natural zeolites, in an attempt to determine their effect on the zeolites crystal structure. The natural Clinoptilolite obtained from Biga-Canakkale region, was stated to be 40-50% pure. The impurities include ilite, montmorinolite, feldspar, calcite, quartz and halite. Stock solutions of cobalt, lead and chromium were prepared in deionised water using the analytical reagent grade cobalt chloride, lead chloride and potassium chromate. The exact concentration of metal ions was verified by AAS.
Adsorption tests were conducted in 250 ml. glass tubes. A zeolite sample of 4.0 gm. was mixed in 100ml. lead, cobalt, and chromium solutions of concentrate ranging from 1 to 100 mg/l by mechanical shaking at a speed of 250 rpm/ min for period of 24hr. The blank experiments were simultaneously carried out without the adsorbent. After the agitation for an equilibrium period, the supernatant solution was filtered through 0.5um micro porous membrane filter.
These first experiments were conducted at room temperature. In the second set of experiments is investigated the influence change chromium and cobalt ions. A accurate weight (4.0 gm.) zeolites sample 1,2 and 3 was mixed and stirred with 100ml. solutions of lead, chromium and cobalt respectively. The investigated initial concentrations were 1,5,10,25,50,75 and 100mg/l. After shaking in a thermostatic system, the solid phase was separated by filtration through a 0.45um micro porous membrane filter. The final PH of solutions was recorded by PH + meter and concentration of lead, chromium and cobalt ions at equilibrium were determined by the atomic adsorption spectrophotometry 20± 0.5°C) (17)
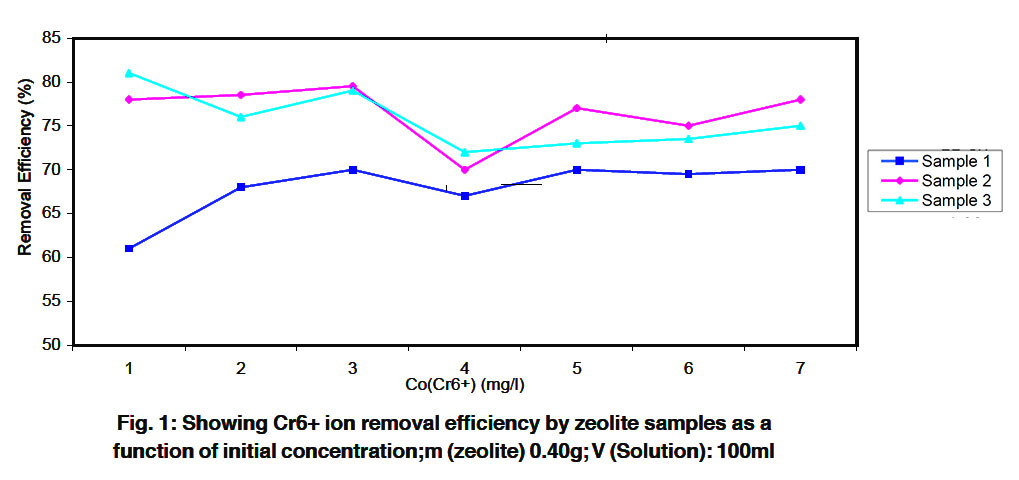 |
Figure 1: Showing Cr6+ ion removal efficiency by zeolite samples as a function of initial concentration;m (zeolite) 0.40g; V (Solution): 100ml Click here to view figure |
Results and Discussion
Chemical analysis of the zeolite is presented I table 1
The lead chromium and cobalt metal ions removal efficiencies for tested zeolite samples are shown in Fig. 1,2 and 3 respectively, 16.80, 19.73, 18.71 mg/g Co2+, 17.86, 19.99, 19.74 mg/g Cr4+ and 10.31, 21.51,23,53 mg/g Pb2+ were taken up by 4.00 g zeolite.
Sample 1,2 and 3. It was clear that, for treated zeolite samples, lead and chromium was more selectively removed than cobalt. The PH value during the experiments was due to the simultaneous uptake of hydrogen ions by zeolite sample and hydrolysis of zeolites.
The removal efficiencies of Pb (II) by the untreated zeolite (SI) are lower than that of the treated zeolite.
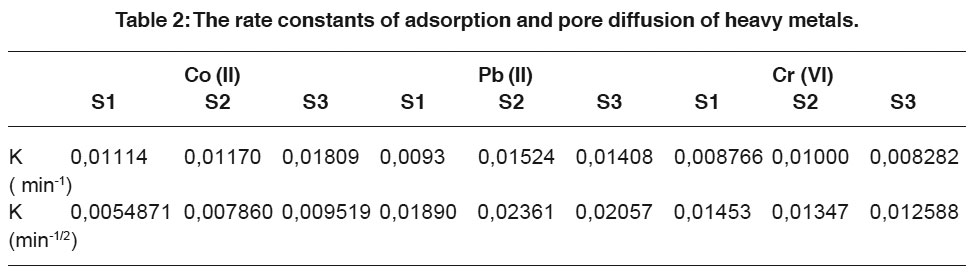 |
Table 2: The rate constants of adsorption and pore diffusion of heavy metals. Click here to view table |
These results indicates that the order of efficiency is as follows: S2>S3>S1 for Co (II) and Pb (II) and S3>S2>S1 for Cr (VI). At cobalt concentration less than 10 mg/l, removal efficiencies of about 94.8% were achieved by all zeolite samples. At higher cobalt concentrations, the removal efficiency decreased to a value between 67,13 and 90% for all zeolite samples 1, 2 and 3 (Fig. 3). In contrast, at higher concentration for Cr (VI) and Pb (II), the removal efficiencies by all samples were increased. However, it was also clear that for all the zeolite samples tested lead was more selectively removed than cobalt.
The rate constant of adsorption pore diffusion and mass transfer coefficient of metal ion were determined using equation of Lagergren and Weber and Morris (Panday K.K., et.al. 1985) respectively which are as follows.
For rate constant of adsorption;
Log (qe-q) = logqe- K/2.3
For rate constant of pore diffusion;
Ct/C0 = Kt 0.5
Where
t; time (min),
q; amount of metal ion adsorbed at time t (mgg-1),
qe; amount of metal ion adsorbed at equilibrium (mgg-1),
C0; initial concentration of metal ion (mgl-1),
Ct; concentration of metal ion a time t (mgg-1),
K; rate constant of adsorption (min-1).
A straight line plot of log (qe-q) Vs t (Fig. 4,5 and 6) suggests the applicability of Lagergren equation, however, the plot of Ct/C0 Vs t0.5 (Fig. 7,8,9), although linear for a wide range of contact period, do not pass through the origin, indicating that the pore diffusion is not the only rate controlling step (Panday K.K., et.al. 1985).
The rate constant of adsorption and pore diffusion were calculated from the slopes of the respective plots and given in table 2
Conclusion
At lead and cobalt concentrations less than 4 mgl-1 removal efficiency was between 80-100% using the untreated zeolites. At the same time concentration of chromium, removal efficiency was about of 70%. It was clear that the lead and cobalt ions were more selectively removed than chromium with the untreated zeolite. But the removal of lead ion is very fast and that finally 92% upon the initial cadmium concentration level. However, the ultimate removal rate remains more or less the same. At lower concentration i.e. concentrations equal to 2.0mg/l or less, rate is lower than the initial concentration except of the S2 zeolite. This shows that the removal of metal ion is highly concentration dependant. It may be noted from the figure that the equilibrium is established in 60 min. and the period of equilibrium is concentration independent. The removal of Pb II. and Co (II) by the treated zeolites increases from 70 to 100%. The rate is not affected by the treatment of zeolite for Cr (VI).
References
- Akyuz T., Akyuz S. and Bassari, A., Journal of Inclusion Phenomena and Macrocyclic Chemistry (2000) 38: 337-344.
- APHA. Standard methods for the examination of water and wastewater, 15th Edition. American Public Health Association, Washington, DC (1985).
- Blanchard G., Maunaye M. and Martin G., Wat. Res. (1984) 18: 1501-1507.
- Chen B., Hui C.W. and McKay G., Water Research., (2000) 35(14): 3345-3356.
- Chen J.P. and Lin M., (2001) 35(10): 2385-2394.
- Curkovic L., Stefanovic S.C. and Filipan T., Wat. Res. (1997) 31(6): 1379-1382.
- Eligwe C.A., Okolue N.B., Nwoko C.I.A., Chem. Engn. Technol. (1999) 22(1): 45-49.
- Juang R.S. and Shao H.J. Water Research (2002) 36(12): 2999-3008.
- Kesraoui-Ouki S., Cheeseman C.R., Perry R., J. Chem. Tech. Bio. (1994) 59: 121-126.
- Kim K.S. and Choi H.C., Wat. Sci. Tech. (1998) 38 (4-5): 95-101.
- Lee D.H. and Moon H., Korean Journal of Chemical Engineering., (2001) 18(2): 247-256.
- Malliou E., Loizidou M. and Spyrellis N., Sci. Total Environ. (1994) 149: 139-144
- Orhan Y. and Buyukgungor H., Wat Sci. Tech. (1993) 28: 247-255.
- Panday K.K., Prasad G .and Singh V.N., Water Res. (1985) 19(7): 869-873.
- Papelis, C., Roberts P.V. and Leckie J.O., Environ. Sci. Technol. (1995) 29: 1099-1108.
- Rao M., Parwate A.V. and Bhole A.G., Waste Management, (2002) 22(7): 821-830.
- Wani P.P, Thorat S.R., Corelation study on physicochemical characteristics of untreated and treated effluents of pulp and paper industry. J. of Current World Environment, (2007) 2(1): 21-26.
- Wani P.P., Sonawane A.C., Thorat S.R., Reduction of chromium by microbial method: transmission electron microscopic study using electron energy loss spectroscopy in-situ environmental cells. J. Pure and Applied Microbiology, (2007)1(2): 331-334.
- Wani P.P., Thorat S.R., Isolating metal tolerance bacteria capable to remove chromium from tannery waste. J. Biosciences, Biotechnology Research Asia, (2007) 4(2): 733-736.
- Wong K.K., Lee, C.K., Low K.S. and Haron M.J., Chemosphere (2003) 50(1): 23-28.
- Yiacoumi S. and Tien C., Kinetics of metal ion adsorption from aqueous solutions: Models algorithms and applications. Kluwer, Norwell M.A (1995).






