Sustainable Management Technique for Recalcitrant Leaf Litter of Mesua Ferrea L. in Avenue Plantations
1
Department of Civil Engineering,
North Eastern Regional Institute of Science and Technology,
Nirjuli,
Arunachal Pradesh
India
2
Department of Botany,
School of Life Sciences, Manipur University,
Canchipur, Imphal,
Manipur
India
Corresponding author Email: linggi32@gmail.com
DOI: http://dx.doi.org/10.12944/CWE.19.1.16
Copy the following to cite this article:
Linggi N, Bharti A, Singh S. Sustainable Management Technique for Recalcitrant Leaf Litter of Mesua Ferrea L. in Avenue Plantations. Curr World Environ 2024;19(1). DOI:http://dx.doi.org/10.12944/CWE.19.1.16
Copy the following to cite this URL:
Linggi N, Bharti A, Singh S. Sustainable Management Technique for Recalcitrant Leaf Litter of Mesua Ferrea L. in Avenue Plantations. Curr World Environ 2024;19(1).
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2023-11-08 |
|---|---|
| Accepted: | 2024-04-15 |
| Reviewed by: | 
 P. N. Krishnan
P. N. Krishnan
|
| Second Review by: |

 Yuva Raj
Yuva Raj
|
| Final Approval by: | Dr. R. K. Aggarwal |
Introduction
Planting trees along the roadsides of highways and pathways is known as avenue plantation1-2. It is practised for enhancing aesthetic value of premises and highways, to provide shade, protect soil erosion, and production of timber, flowers and fruits. The plantations also provide a sink for carbon dioxide3-5, release fresh oxygen6 and improve soil fertility. Avenue trees are commonly planted along roadsides in institutional campuses, commercial complexes, government establishment premises and residential areas, according to National Forest Policy (1952). A few of the most preferred tree species used for avenue plantations include Saraca indica (Ashoka tree), Delonix regia (Gulmohar), Messua ferrea L. (Nahar), Mimusops elengi (Maulsari/ Bakul), Anthocephalus cadamba (Kadam), Elaeocarpus granitus (Rudraksha), Terminalia chebula (Arjuna) and Gravillea robusta7. Generally, perennial and evergreen tree species are selected for avenue plantations since they produce green leaves throughout the year and possess an ornamental tree canopy and colourful flowers.
Although avenue plantations provide multiple benefits, they also pose a major management problem due to the accumulation of a significant amount of leaf litter8. The most widely adopted management practice for leaf litter in avenue plantations involves either disposal through municipal waste management systems or burning in the corner of the premises or at the collection site along plantation areas, which is not a standard protocol prescribed under Solid Waste Management Rules (2016). It is well known that the burning of leaf litter causes the immediate release of various gases such as CO2, CO, SO2 and other airborne particulate matters in the atmosphere, thereby enhancing global warming and air pollution9-10.
The natural decomposition of leaf litter from certain tree species such as Messua ferrea L., Ailanthus grandis, Castanopsis indica, and Vatica lancefolia has been reported to be slower as compared to other leaf litter due to the presence of higher content of lignin, lower nitrogen contents11-14 and presence of phenolic compounds in the leaf tissues15. The major problem of MF leaf litter is recalcitrance, leading to very slow natural decomposition with an average life span of three years. Naturally, microorganisms decompose leaf litter under suitable environmental conditions16 and obtain nutrients such as C, N, P, Ca, Mg, etc. They play major roles in nutrient recycling by releasing these nutrients back into the soil17-21 through enzyme-catalyzed reactions during the process of plant litter decomposition22-24. Among microorganisms, bacteria and fungi are known to decompose cellulose25, while fungi are the main decomposers of cellulosic matter by releasing cellulase enzymes26-27.
The need to recycle organic wastes to enhance soil fertility has increased in recent years due to the high cost of fertilizers and decreased availability of organic manures28-29. Despite widespread avenue plantations inside the institutional campuses, premises of government establishments and commercial complexes, no sustainable leaf litter management practices have been developed with reference to a specific plant species. Therefore, the present study aimed to evaluate the decomposition rate of MF leaf litter in natural conditions and evaluate nutrient dynamics for developing an effective and sustainable management strategy.
Material and methods
Study Site
The present study was conducted in the avenue plantation inside the campus of North Regional Institute of Science and Technology (NERIST), Deemed-to-be-University, Nirjuli, Arunachal Pradesh. The campus avenue plantation is dominated by more than 160 evergreen trees of Mesua ferrea L.(MF) trees, commonly known as Nahar or Nageshwar (Hindi), along the roadsides, covering an area of 2.34 hectares within the campus. The MF tree has a beautiful canopy and remains covered with green foliage throughout the year, producing considerable leaf litter (solid biomass).
Collection of leaf litter and preparation of litterbags
Freshly fallen MF leaf litter was collected by constructing a green net trap under the tree canopies to avoid exposure to the soil surface. The MF leaf litter was oven-dried at 60°for 48h. The oven-dried MF leaf litter was divided into two parts: intact (without shredding the leaves) and shredded (by breaking down into 2-3 cm-sized fragments). For the control treatment, intact MF leaf litter was used. The litterbags of uniform size (20 cm x 20 cm) to hold 10g of MF leaf litter were prepared using a nylon net having a mesh size of 2 mm.
Decomposition of MF leaf litter
The decomposition rate and nutrient dynamics of the MF leaf litter were studied in natural conditions using the modified litterbag method3. A total of six (6) pits of 1 ft2 were dug out by keeping a minimum distance of 1 metre apart between 2 pits within the institute campus. The pits were embedded with 6 litterbags (3 replicates of intact and 3 replicates of shredded MF leaf litter). Thus, a total of 36 (6 x 6) litterbag samples were prepared to be embedded in the soil. After embedding in the pits, the litterbags were covered with a layer of soil, marked by a labelled peg, to enable identification of the exact location of the litterbags during sample collection. Close to the vicinity of the embedded litterbags, three control plots, each 1m2 were prepared by spreading the intact MF leaf litter (150 g) on the ground surface of the soil to enable a natural decomposition process without embedding in the soil. The control plots were well protected by erecting agro-net fences and roofs. The litterbag samples and control samples were studied for a year (360 days) and periodically removed for analysis.
Sample collection and analysis
The litterbag samples were recovered from each pit after 60, 120, 180, 240, 300 and 360 days, respectively. The recovered litterbag samples were carefully put in sterile polythene bags and brought to the laboratory for analysis. Subsamples weighing 5 gm were taken from the litterbags and stored in a refrigerator at a temperature of 4° C for microbial analysis. Unlike the litterbag samples, the control samples were left undisturbed and recovered at the end of 360 days.
Determination of biomass loss
The recovered litterbags from the pits were washed in a plastic bucket or tub with tap water by swirling briefly and carefully decanting through a 2 mm mesh size sieve to remove extraneous matter such as soil, roots and sediments. Such brief washing allows the leaching of nutrients31. Then, cleaned MF leaf litter samples were drained off to remove excess water and oven-dried at 60°C for 48 hours, and then the dry weight was recorded. An attempt was also made to find out the statistical significance of the biomass loss with respect to time using a simple linear regression equation (Y = a + bx) 32.
Nutrient content in MF leaf litter
The oven-dried MF leaf samples were grounded to a fine powder (60 mesh sieve < 0.25 mm). The nutrient contents of the MF leaf litter sample (250 mg) were analyzed from the aliquots obtained from the acid digestion of the samples according to the standard methods33-34. The total nitrogen (TN) was determined using a micro-Kjeldahl distillation method and total phosphorus (TP) using the colourimetric method with pH adjustment. The total organic carbon (TOC) content was obtained by hot digestion of the powdered MF leaf litter (50 mg) with concentrated sulphuric acid (H2SO4) and an aqueous potassium dichromate (K2Cr2O4) solution, followed by titration with ferrous ammonium sulphate [(NH4)2Fe(SO4)2·6H2O] solution.
Estimation of annual decay constant (k)
Olson's (1963) decay model35 has been employed to assess the differences in MF leaf litter decomposition rates for the control, intact and shredded leaf litter. According to this model decay constant (k) was calculated as follows:
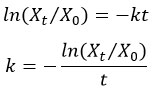
Where
X0 = Initial dry weight,
Xt = Remaining dry weight after the time (t),
t = Time (year), ln = Natural log
The time required for the MF leaf litter to decompose, 50% (t1/2) and 99% (t99) of its dry weight, were calculated as follows;
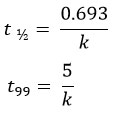
The decay constant (k) value directly corresponds to the decomposition rate, i.e., a higher k value implies a higher decomposition rate. In addition, a higher k value will have lesser t1/2 and t99, implying that the time required for decomposition will be less.
Determination of Nutrient Released
The nutrient released from the MF leaf litter during decomposition was calculated (%) as minus nutrient remaining (NR) from 100 per cent at the time of sampling36:
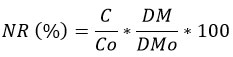
Nutrient Released (%) = 100 - NR(%)
Where
NR (%) = Nutrient Remaining
C = Concentration of element at the time of sampling,
Co = Initial Concentration of the element prior to decomposition,
DM = Dry mass at the time of sampling and
DMo = Initial dry mass of the sample prior to decomposition.
Screening of cellulase activity for isolated fungi
Five-gram (5gm) subsamples from the litterbags were suspended in 95 ml sterilized distilled water, followed by serial dilution up to 10-3. A volume of 100 µl of inoculum from the 10-3 dilution was evenly spread on the solidified potato dextrose agar (PDA) media plates and incubated at 28ºC for 4-7 days to isolate fungi. Pure fungal isolates were obtained by sub-culture of fungal isolates from mixed plates on fresh PDA plates and slants and stored at 4ºC.
Cellulase activity was tested using Mendel and Reese Agar (MRA) media37 supplemented with 0.5% Carboxymethyl cellulose Sodium salt (CMC-Na) containing KH2PO4 (2g), NH4SO4 (1.4g), MgSO4.7H2O (0.3g), CaCl2 (0.3g), Yeast extract (0.4g), FeSO4.7H2O (0.005g), MnSO4 (0.0016g), ZnCl2 (0.0017g), CoCl2 (0.002g), CMC-Na (5g), Agar (5g), distilled water (1000 ml) and final pH adjusted to 5±0.2. The purified fungal isolates were inoculated in the petri dish containing 20 ml of MRA CMC-Na media and incubated at 28ºC for 4-7 days until the growth reaches a minimum diameter of 1-2 cm. After attaining the desired growth, the plates were flooded with 10 ml of 1% Congo red solution for staining38 and were allowed to stand for a minimum of 15 minutes. The excess Congo-red stain was drained off, and the plates were de-stained by flooding with 1M Sodium chloride (NaCl) solution for 30 minutes. After de-staining, NaCl was discarded, and the plates were analyzed for the formation of the hydrolyzing zone (clear zone/ halo zone) surrounding the colonies, which indicates the breakdown of CMC-Na by the fungal isolates via the release of cellulase39-41.
Hydrolyzing index (HI)
The fungal isolates with cellulolytic activities were selected, and their hydrolyzing index (HI) was calculated. The HI is also termed as the Index of Relative Enzyme Activity expressed as the ratio of the hydrolyzing zone diameter to the colony diameter42-43. The greater HI value corresponds to the higher cellulase production/ enzymatic activity of the fungus.
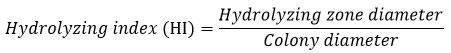
Identification of cellulase-producing fungi
The fungal isolates confirming cellulase activity were identified by examining the morphological characteristics with the help of microscopic images up to the genus and species level using various fungal identification manuals44-45 and fungal identification databases such as http://www.indexfungorum.orgn and http://www.mycokey.com.
Results
Biomass loss pattern
After 360 days of the decomposition period, the lowest amount of biomass (13.97 %) was recorded for shredded leaf litter, followed by intact (14.70%), and the control showed the highest biomass (71.70%), as shown in Figure 1.
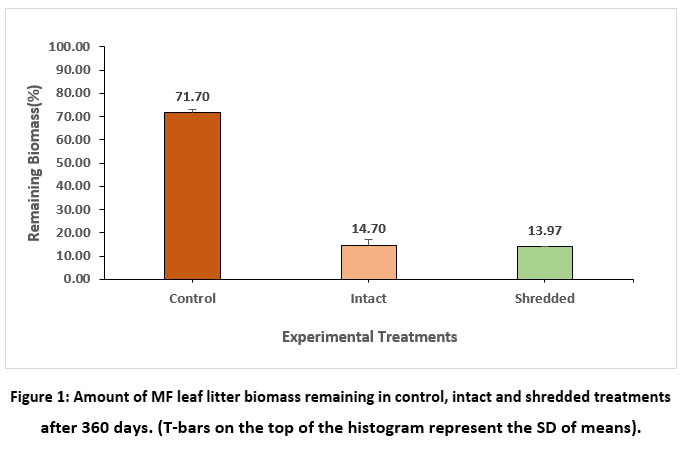 | Figure 1: Amount of MF leaf litter biomass remaining in control, intact and shredded treatments after 360 days. (T-bars on the top of the histogram represent the SD of means).
|
The extensive study of the effect of shredding MF leaf litter using the modified litterbag method showed a three-phased pattern of biomass loss for both intact and shredded states. Also, a strong positive correlation was observed between biomass loss with time for intact (r2=0.936; p =0.001) and shredded (r2=0.935; p =0.001) MF leaf litter. In the first phase of the study, i.e., upto120 days period, a rapid decline in biomass was observed, and 50% of the total litter biomass was lost for both intact and shredded states [Figure 2]. However, the second phase of decomposition from 120-180 days displayed a very slow rate for both intact and shredded MF leaf litter. During the third phase of decomposition, between 180 to 360 days, both intact and shredded MF leaf litter showed a rapid loss of biomass, as shown by the loss of more than 80% of the biomass.
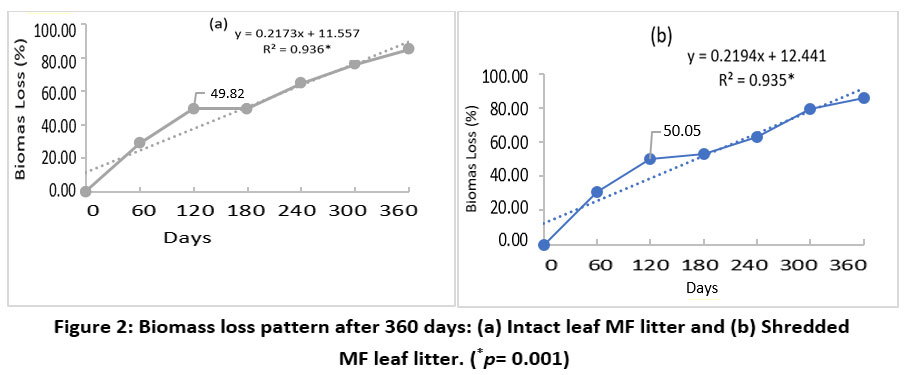 | Figure 2: Biomass loss pattern after 360 days: (a) Intact leaf MF litter and (b) Shredded MF leaf litter. (*p= 0.001)
|
Based on the biomass loss, the rate of decomposition was calculated (Table 1), and the shredded MF leaf litter showed a slightly higher value (0.029 ± 0.016 g/day) than the intact state value (0.028 ± 0.015 g/day).
Table 1: Biomass Decomposition rate (g/day) of Intact and Shredded MF leaf litter.
Biomass Decomposition Rate (g/day) | |
| Intact (±SD) | Shredded (±SD) |
| 0.028±0.015 | 0.029±0.016 |
Percentage of Nutrients Released
The soil in the experimental plot was slightly acidic pH (6.13±0.79), and the total organic carbon (TOC), total nitrogen (TN) and total phosphorus (TP) contents were 2.31±0.30 %, 0.15±0.02% and 0.13±0.06% respectively. The percentage of nutrients released after 360 days of decomposition was calculated for intact and shredded MF leaf litter with respect to their initial biomass, nutrient content and remaining biomass. The total organic carbon (TOC%), total nitrogen (TN%) and total phosphorus (TP%) release followed a similar pattern (TOC>TN>TP) for both intact and shredded MF leaf litter (Table 2). However, it was observed that the shredded MF litter showed a marginally higher amount of release of nutrients than intact MF litter.
Table 2: Percentage of nutrients released by intact and shredded MF leaf litter at the end of 360 days of decomposition.
| Parameters | Nutrients Released (%) | |||
| Intact | ± SD | Shredded | ± SD | |
| TOC (%) | 86.80 | 3.88 | 87.20 | 4.10 |
| TP (%) | 86.60 | 1.71 | 88.54 | 0.32 |
| TN (%) | 75.10 | 2.46 | 77.11 | 6.33 |
Annual Decay Constant (k)
The decay constant of MF leaf litter was calculated at the end of 360 days with respect to the final remaining biomass. It was observed that the shredded leaf litter had the highest k value (1.99) as compared to the intact (1.92), while the control had the lowest k value (0.33). The comparison of the k, t50 and t99 values with other avenue tree species shows that intact MF leaf litter (control) has the lowest k value (0.33), suggesting that it will require the longest time (t99) to decompose 99% of its biomass in natural conditions (Table 3).
Table 3: Decay constant (k), t50 and t99 values for MF leaf litter and other avenue tree species.
| Type of leaf litter | Decay Constant (k) | t50 (year) | t99 (year) | References |
| Intact MF (Control) | 0.33 | 2.08 | 15.03 | Present study |
| Intact MF | 1.92 | 0.36 | 2.60 | |
| Shredded MF | 1.99 | 0.35 | 2.51 | |
| MF | 0.80 | 0.86 | 6.22 | Arunachalam and ND Singh, 2004 |
| Tectona grandis | 1.27 | 0.54 | 3.92 | Jha, 2010 |
| Pinus kesiya | 1.28 | 0.50 | 3.90 | Arunachalam, 1998 |
| Quercus dealbata | 0.88 | 0.80 | 5.70 | |
| Quercus griffithii | 1.39 | 0.50 | 3.60 | |
| Rhododendron arboreum | 0.77 | 0.90 | 6.50 | |
| Sterculia khasiana | 1.06 | 0.70 | 4.70 |
Isolation, screening and identification of cellulase-producing fungi
A total of 170 fungal colony-forming units (CFUs) were isolated from decomposing MF leaf litter samples during the decomposition period. Eighteen (18) isolates showed cellulose-degrading activity, as indicated by the presence of hydrolyzing zones on the culture plates (Table 4).
Table 5: List of fungal isolates showing cellulase degrading activity. (low activity = +, moderate activity = ++, high activity= +++/++++)
| Sl. No. | Isolate No. | Genus/ Species | HI (±SD) | Cellulase activity |
| 1. | F-2 | Penicillium sp.1 | 1.42 ± 0.05 | + |
| 2. | F-7 | Penicillium sp.2 | 1.31 ± 0.02 | + |
| 3. | F-8 | Penicillium brevicompactum | 1.46 ± 0.01 | + |
| 4. | F-15 | Mucor sp. | 1.17 ± 0.00 | + |
| 5. | F-16 | Penicillium sp.3 | 1.30 ± 0.09 | + |
| 6. | F-20 | Unidentified 1 | 1.28 ± 0.16 | + |
| 7. | F-39(A) | Penicillium sp.4 | 3.54 ± 0.47 | +++ |
| 8. | F-60 | Penicillium sp.5 | 1.34 ± 0.08 | + |
| 9. | P-2/F-11 | Unidentified 2 | 1.20 ± 0.06 | + |
| 10. | P-2/F-28 | Penicillium decumbens | 2.60 ± 0.57 | ++ |
| 11. | P-6/F-15(A) | Aspergillus niger | 4.25 ± 0.25 | ++++ |
| 12. | P-6/F-15(B) | Penicillium chrysogenum | 1.09 ± 0.06 | + |
| 13. | V-3/F-3 | Fusarium sp. | 1.09 ± 0.04 | + |
| 14. | V-3/F-4 | Penicillium sp.6 | 2.70 ± 0.36 | ++ |
| 15. | V-3/F-17(A) | Penicillium perpurogenum | 3.06 ± 0.42 | +++ |
| 16. | V-3/F-22 | Penicillium sp.7 | 1.95 ± 0.49 | + |
| 17 | V-3/F-25 | Penicillium variabile | 1.62 ± 0.03 | + |
| 18. | BR-II-A/5 | Unidentified 3 | 1.35 ± 0.05 | + |
The identified fungal isolates belonged to 4 genera: Aspergillus, Fusarium, Mucor and Penicillium. Six (6) species were identified up to the species level, 9 were identified up to the genus level, and 3 were unidentified species. Aspergillus niger showed a very high HI value of (4.25±0.25) followed by Penicillium perpurogenum and Penicillium sp. 4 with an HI value of (3.54±0.47) and (3.06±0.42) respectively. Penicillium sp.5 and Penicillium decumbens showed moderately high HI values (2.70±0.36) and (2.60±0.57), while the rest of the isolates showed lower HI values.
Discussion
A considerable amount of leaf litter is produced by Mesua Ferrea L. (MF) trees growing as avenue plantations throughout the year. The leaves are recalcitrant to the natural decomposition process and remain for a long time on the surface of the ground. The decomposition of MF leaf litter in natural conditions using the modified litterbag embedding method revealed that shredded leaf litter showed a slightly higher decomposition rate as compared to intact leaf litter. In contrast, leaf litter under controlled conditions (unembedded) has shown a significantly slower decompaction rate, as revealed by a high amount of biomass remaining undecomposed over 1 year. In the present study, it was found that significantly lower amounts of biomass remained at the end of 360 days, as shown by shredded (13.97%) and intact (14.7%) MF leaf litter. A study conducted on MF leaf litter decomposition in natural conditions by using the modified litterbag method revealed that 46.60% of biomass remained at the end of 360 days46. However, in their study, the embedded litterbags were covered with MF leaf litter as compared to the present study, in which the litterbags were covered with a layer of soil. Rapid decomposition in the litterbags could be attributed to the availability of moisture and the abundance of diverse microbial communities in the soil. The litterbags were embedded during the monsoon month (August) characterized by frequent rainfall, high temperature and humidity. As reported by various researchers, temperature and moisture are the two major factors that affect the decomposition rate47-50. However, the rate of decomposition was very slow in the case of control treatment where leaf litter was openly scattered on the soil surface, exposed to normal environmental conditions.
The comparative study of the decomposition of intact and shredded states of MF leaf litter in the litterbags showed a marginal difference following three-phased decomposition patterns, i.e. initial rapid decomposition up to 4 months (120 days) followed by a slow decomposition phase between 4 to 6 months (120-180 days) and a rapid decomposition phase after 6 months to 12 months (180-360 days). In the first phase of decomposition, the decline in biomass loss was rapid50-53 could be due to rapid microbial decomposition and absorption of nutrients, especially the water-soluble components and simple substrates54. The second phase showed no significant biomass loss, which may be due to the recalcitrant fraction (lignin) of the leaf litter55-56. During the third phase of decomposition, both intact and shredded MF leaf litter showed a more or less uniform pattern of biomass loss up to 360 days, which could be attributed to the acclimatization of microbes capable of decomposing recalcitrant fractions over time. In addition, the decreased decomposition rate during winter could be due to cold and insufficient moisture availability and reduced microbial activities57-59. The findings of this study assume that the smaller sizes of leaf litter in shredded leaf litter mixed with soil materials could have provided suitable micro-environmental conditions for the growth and reproduction of microorganisms, leading to the degradation of leaf tissues. The rate of decomposition might have been enhanced since most microbial invasions occur near or on the surface of leaf tissues60. A similar observation of a three-phased decomposition pattern was also reported for MF leaf litter; however, the initial stage of their investigation exhibited a moderate level of decomposition lasting up to 90 days, which was then followed by a gradual decomposition phase lasting up to 180 days and finally a rapid decomposition period lasting up to 360 days46.
Based on decay constant (k) values, the shredded leaf treatment required less time (approx. 0.35 years) to decompose 50% of its biomass as compared to the intact (0.36 years). The k value of the control treatment showed the highest time of 2.51 years required for the decomposition of 50% of the biomass. The time (t) required to decompose 99% of the biomass will be 2.51, 2.6 and 15.03 years for the shredded, intact and controlled leaf litter of MF, respectively. The k values of intact and shredded leaf litter of MF in this study were significantly higher than the same species and other avenue tree species reported by different studies46,61. According to the nutrient dynamic analyses, the nutrients released were observed in the pattern of TOC>TP>TN for both intact and shredded states. However, each nutrient released value was slightly higher for the shredded leaf litter state as compared to the intact state. A similar study conducted by Jha (2010)61 on Tectona grandis leaf litter decomposition reported that the nutrients released flow pattern was TOC>TN>TP.
Screening for cellulose-degrading activity revealed that Penicillium was found as the dominant fungal genus with 12 isolates. Aspergillus niger showed the highest cellulase activity with the HI value of 4.25±0.25, followed by Penicillium perpurogenum with 3.06±0.42. Previous studies have also reported cellulose-degrading activities of different species of Aspergillus and Penicillium, particularly Aspergillus niger and Penicillium perpurogenum 62-65.
Conclusion
In the present study, the decomposition rate of MF leaf litter has been found to occur at a faster rate by applying the modified litterbag method where the litterbags were covered with a layer of soil. After 360 days of the decomposition period, the embedded litterbags, i.e., shredded and intact MF leaf litter, were found to decompose rapidly as compared to non-embedded leaf litter spread on the surface of the soil as control. The shredded and intact MF leaf litter displayed higher values of decay constant (k), indicating that decomposing 99% of biomass will require 2.5 years and 2.6 years, respectively, using the proposed litterbag method. However, the lower decay constant (k) value of the control MF leaf litter indicated that the decomposition of 99% biomass naturally would require a very long period of approximately 15.03 years. The presence of dominant cellulose-decomposing filamentous fungi like Aspergillus and Penicillium might have played an important role in the faster decomposition of MF leaf litter. Based on the findings of the present study, it can be concluded that proper management of the leaf litter generated by MF in avenue plantations can be accomplished by burying the leaf litter in a suitable area of the avenue plantation premises instead of burning it. The decomposed biomass in the form of compost or manure produced within 4 months may be utilized for the horticultural, floricultural and agricultural soil management practices of campus and other agricultural lands. In addition to enhancing aesthetic values, this sustainable management technique may reduce air pollution caused by burning leaf litter and reduce applying chemical fertilizers in horticultural and floricultural activities on academic and government premises in the long term.
Acknowledgement
We thank the Dept. of Forestry NERIST, for providing laboratory facilities in order to complete the experimental work in time successfully. We would also like to thank Prof. Karuna Shrivastava for her valuable suggestions and feedback throughout the study period and the two anonymous reviewers for their valuable comments and suggestions in giving this manuscript a final shape.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflicts of Interest
The authors declare no conflict of interest.
Author's Contribution
Nirigi Linggi conducted the experiment, collected and analyzed data and she has also drafted the manuscript of this research work.
S. Sureshkumar Singh has developed the concept, designed the experiments and edited the manuscript of the present research work.
Ajay Bharti has also been involved in experimental design, data analysis and editing the manuscript of the research work.
Data Availability
There is no additional data available for the present research work.
Ethics Approval Statement
Present study did not involved any experiment on humans or animals.
References
- Rao MS. Introduction to Social Forestry. Oxford & IBH Publishing Co. Pvt. Ltd., New Delhi, India; 1979.
- Singh AK, Singh NP. Agricultural Terminology. Concept Publishing Company; 2004.
- Chavan BL, Rasal GB. Sequestered standing carbon stock in selective tree species grown in University campus at Aurangabad, Maharashtra, India. Int J Eng Sci Technol. 2010;2(7):3003-3007.
- Jo HK. Impacts of urban greenspace on offsetting carbon emissions for middle Korea. J Environ Manage. 2002;64(2):115-126.
- Johnson AD, Gerhold HD. Carbon storage by urban tree cultivars, in roots and above-ground. Urban For Urban Green. 2003;2(2):65-72.
- McPherson G, Simpson JR, Peper PJ, Maco SE, Xiao Q. Municipal forest benefits and costs in five US cities. J For. 2005;103(8):411-416.
- Pangging G, Pongen M. Avenue plantation in eastern Himalaya: A case study of avenue trees in NERIST campus, Arunachal Pradesh. Int J Plant Anim Environ Sci. 2015;5(4):73-75.
- Gandherva D, Bhattacharya P. Collection and Quantification of Leaf Litter from Urban Street Plantation: A Case Study on South-West Delhi, India. In: Sustainable Climate Action and Water Management. Singapore: Springer Singapore; 2021:275-282.
- Ward DE, Setzer AW, Kaufman YJ, Rasmussen RA. Characteristics of smoke emissions from biomass fires of the Amazon region base-A experiment. 1991;394-402.
- Abbasi SA. Environmental pollution and its control: a reader-friendly introduction to environmental engineering. 1999.
- Arunachalam A, Maithani K, Pandey HN, Tripathi RS. Leaf litter decomposition and nutrient mineralization patterns in regrowing stands of a humid subtropical forest after tree cutting. Forest Ecology and Management. 1998;109(1-3):151-161.
- Laishram ID, Yadava PS. Lignin and nitrogen in the decomposition of leaf litter in a subtropical forest ecosystem at Shiroy hills in northeastern India. Plant and Soil. 1988;106:64.
- Hobbie SE. Interactions between litter lignin and soil nitrogen availability during leaf litter decomposition in a Hawaiian montane forest. Ecosystems. 2009;(3):484-494.
- Gessner MO, Swan CM, Dang CK, McKie BG, Bardgett RD, Wall DH, Hattenschwiler S. Diversity meets decomposition. Trends in ecology & evolution. 2010;25(6):372–380.
- Bernhard-Reversat F, editor. Effect of exotic tree plantations on plant diversity and biological soil fertility in the Congo savanna: with special reference to eucalypts. CIFOR; 2001.
- Vossbrinck CR, Coleman DC, Woolley TA. Abiotic and biotic factors in litter decomposition in a semiarid grassland. Ecology. 1979;60(2):265-271.
- Singh KP, Singh PK, Tripathi SK. Litterfall, litter decomposition and nutrient release patterns in four native tree species raised on coal mine spoil at Singrauli, India. Biol Fertil Soils. 1999;(29):371-378.
- Silveira M, Reddy K, Comerford N. Litter Decomposition and Soluble Carbon, Nitrogen, and Phosphorus Release in a Forest Ecosystem. Open J Soil Sci. 2011;1(3):86-96.
- Mudrick DA, Hoosein M, Hicks Jr RR, Townsend EC. Decomposition of leaf litter in an Appalachian Forest: effects of leaf species, aspect, slope position and time. Forest Ecol Manage. 1994;68(2-3):231-250.
- Aber JD, Melillo JM. Terrestrial ecosystems (Vol. 429). Philadelphia: Saunders College Publishing; 1991.
- Berg B, McClaugherty C. Nitrogen and phosphorus release from decomposing litter in relation to the disappearance of lignin. Can J Bot. 1989;67(4):1148-1156.
- Romani AM, Fischer H, Mille-Lindblom C, Tranvik LJ. Interactions of bacteria and fungi on decomposing litter: differential extracellular enzyme activities. Ecology. 2006;87(10):2559-2569.
- B?onska E, Piaszczyk W, Staszel K, Lasota J. Enzymatic activity of soils and soil organic matter stabilization as an effect of components released from the decomposition of litter. Appl Soil Ecol. 2021;157:103723.
- Li J, Wang P, Yang J, Guan P. Soil degradation regulates the effects of litter decomposition on soil microbial nutrient limitation: Evidence from soil enzymatic activity and stoichiometry. Front Plant Sci. 2023;13:1090954.
- Lederberg J. Encyclopedia of microbiology. Academic Press; 2000.
- Khokhar I, Haider MS, Mushtaq S, Mukhtar I. Isolation and screening of highly cellulolytic filamentous fungi. J Appl Sci Environ Manage. 2012;16(3).
- Lynd LR, Weimer PJ, van Zyl WH, Pretorius IS. Microbial cellulose utilization: fundamentals and biotechnology. Microbiol Mol Biol Rev. 2002;66(3):506–577.
- Sharma A, Sharma A, Singh S, Vig AP, Kaur NA. Leaf litter vermi composting: converting waste to resource. IOP Conf Ser Earth Environ Sci. 2021 Nov;889(1):012066.
- Revs IJMR. Efficiency of Perionyx excavatus (Perrier) in litter (Anacardium occidentale L.) decomposition and nutrient mineralization. Int J Modn Res Revs. 2014;2(10):453-458.
- Gilbert O, Bocock KL. Changes in the leaf litter when placed on the surface of soils with contrasting humus types. II. Changes in the nitrogen content of oak and ash litter. J Soil Sci. 1960;(11):10–19.
- Anderson JM, Ingram JS. Tropical soil biology and fertility: a handbook of methods. Soil Science. 1994;157(4):265.
- Zar JH. Biostatistical Analysis. Englewood Cliffs, NJ: Prentice-Hall; 1974.
- Allen SE, Grimshaw HM, Parkinson JA, Quarmby C. Chemical analysis of ecological materials. Blackwell Scientific Publications; 1974.
- Okalebo JR, Gathua KW, Woomer PL. Fractionation of organic matter by particle size. 1993.
- Olson JS. Energy storage and the balance of producers and decomposers in ecological systems. Ecology. 1963;44(2):322-331.
- Bockheim JG, Jepsen EA, Heisey DM. Nutrient dynamics in decomposing leaf litter of four tree species on a sandy soil in northwestern Wisconsin. Can J For Res. 1991;21(6):803-812.
- Mandels M, Hontz L, Nystrom J. Enzymatic hydrolysis of waste cellulose. Biotechnol Bioeng. 1974;16(11):1471-1493.
- Teather RM, Wood PJ. Use of Congo red-polysaccharide interactions in enumeration and characterization of cellulolytic bacteria from the bovine rumen. Appl Environ Microbiol. 1982;43(4):777-780.
- Lu WJ, Wang HT, Nie YF, Wang ZC, Huang DY, Qiu XY, Chen JC. Effect of inoculating flower stalks and vegetable waste with ligno-cellulolytic microorganisms on the composting process. J Environ Sci Health B. 2004;39(5-6):871–887.
- Lakshmanan R, Muthunarayanan V. Enzymatic analysis of Natural and Artificial Banana Leaf waste in Vermi-composting and Composting Technique. J Adv Appl Sci Res. 2016;1(6):50-61.
- Almuharef I, Rahman MS, Qin W. High production of cellulase by a newly isolated strain Paenibacillus sp. IM7. Waste Biomass Valor. 2020;11:6085-6094.
- Bradner J, Gillings M, Nevalainen K. Qualitative assessment of hydrolytic activities in antarctic microfungi grown at different temperatures on solid media. World J Microbiol Biotechnol. 1999;15:131–132.
- Peciulyte D. Isolation of cellulolytic fungi from waste paper gradual recycling materials. Ekologija. 2007;53(4).
- Domsch KH, Gams W, Anderson TH. Compendium of soil fungi. Volume 1. Academic Press (London) Ltd; 1980.
- Nagamani A, Kunwar IK, Manoharachary C. Handbook of Soil Fungi. 2006.
- Arunachalam A, Singh ND. Decomposition of Mesua ferrea litter in humid tropics of Arunachal Pradesh, India. J Trop For Sci. 2004;16(2):151-159.
- Agbim NN. Dry Season Decomposition of Leaf Litter from Five Common Plant Species of West Africa. Biol Agric Hort. 1987;(4):213-224.
- Paul K. Temperature and moisture effects on decomposition. Net ecosystem exchange. 2001;95-102.
- Sierra CA, Malghani S, Loescher HW. Interactions among temperature, moisture, and oxygen concentrations in controlling decomposition rates in a boreal forest soil. Biogeosciences. 2017;14(3):703-710.
- Petraglia A, Cacciatori C, Chelli S, Fenu G, Calderisi G, Gargano D, et al. Litter decomposition: effects of temperature driven by soil moisture and vegetation type. Plant Soil. 2019;435:187-200.
- Anderson JM, Proctor J, Vallack HW. Ecological Studies in Four Contrasting Lowland Rain Forests in Gunung Mulu National Park, Sarawak: III. Decomposition Processes and Nutrient Losses from Leaf Litter. J Ecol. 1983;71(2):503–527.
- Kumar BM, Deepu JK. Litter production and decomposition dynamics in moist deciduous forests of the Western Ghats in Peninsular India. For Ecol Manage. 1992;50(3-4):181-201.
- Jama BA, Nair PKR. Decomposition-and nitrogen-mineralization patterns of Leucaena leucocephala and Cassia siamea mulch under tropical semiarid conditions in Kenya. Plant Soil. 1996;179:275-285.
- Songwe NC, Okali DUU, Fasehun FE. Litter decomposition and nutrient release in a tropical rainforest, Southern Bakundu Forest Reserve, Cameroon. J Trop Ecol. 1995;11(3):333-350.
- Bloomfield J, Vogt KA, Vogt DJ. Decay rate and substrate quality of fine roots and foliage of two tropical tree species in the Luquillo Experimental Forest, Puerto Rico. Plant Soil. 1993;150:233-245.
- Gaudinski JB, Trumbore SE, Davidson EA, Zheng SH. Soil carbon cycling in a temperate forest: radiocarbon-based estimates of residence times, sequestration rates and partitioning of fluxes. Biogeochemistry. 2001;52(1):113-114.
- Sarjubala Devi A, Yadava PS. Wood and leaf litter decomposition of Dipterocarpus tuberculatus Roxb. in a tropical deciduous forest of Manipur, Northeast India. Curr Sci. 2007;93(2).
- Du T, Zhang L, Chen Y, Zhang Y, Zhu H, Xu Z, et al. Decreased snow depth inhibits litter decomposition via changes in litter microbial biomass and enzyme activity. Sci Total Environ. 2024;921:171078.
- Schimel JP, Gulledge JM, Clein-Curley JS, Lindstrom JE, Braddock JF. Moisture effects on microbial activity and community structure in decomposing birch litter in the Alaskan taiga. Soil Biol Biochem. 1999;31(6):831-838.
- Verma SL, Marschner P. Compost effects on microbial biomass and soil P pools as affected by particle size and soil properties. J Soil Sci Plant Nutr. 2013;13(2):313–328.
- Jha KK. Litter Production and Leaf Litter Decomposition Dynamics in an Age Series Tectona Grandis Linn. E Plantations of Moist Tara Sal Forest. Indian For. 2010;136(1).
- Hurst PL, Nielsen JAN, Sullivan PA, Shepherd MG. Purification and properties of a cellulase from Aspergillus niger. Biochem J. 1977;165(1):33-41.
- Baldrian P, Gabriel J. Lignocellulose degradation by Pleurotus ostreatus in the presence of cadmium. FEMS Microbiol Lett. 2003;220(2):235-240.
- Dhake AB, Patil MB. Production of ß-Glucosidase by Penicillium purpurogenum. Braz J Microbiol. 2005;(36):170-176.
- Jeya M, Joo AR, Lee KM, Tiwari MK, Lee KM, Kim SH, Lee JK. Characterization of ?-glucosidase from a strain of Penicillium purpurogenum KJS506. Appl Microbiol Biotechnol. 2010;86:1473-14.






