Efficacy of Modified Magnolia champaca Bark Powder in Sequestration of Divalent Ions from Aqueous Matrices
1
Department of Chemistry,
PSGR Krishnammal College for Women,
Peelamedu, Coimbatore,
Tamil Nadu
India
Corresponding author Email: muthulakshmiandal@psgrkcw.ac.in
DOI: http://dx.doi.org/10.12944/CWE.19.1.6
Copy the following to cite this article:
Andal N. M, Indhumathy P. Efficacy of Modified Magnolia champaca Bark Powder in Sequestration of Divalent Ions from Aqueous Matrices. Curr World Environ 2024;19(1). DOI:http://dx.doi.org/10.12944/CWE.19.1.6
Copy the following to cite this URL:
Andal N. M, Indhumathy P. Efficacy of Modified Magnolia champaca Bark Powder in Sequestration of Divalent Ions from Aqueous Matrices. Curr World Environ 2024;19(1).
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2023-10-27 |
|---|---|
| Accepted: | 2024-03-27 |
| Reviewed by: | 
 Maphuti Kwata
Maphuti Kwata
|
| Second Review by: |

 Ponnusamy Thillaiarasu
Ponnusamy Thillaiarasu
|
| Final Approval by: | Dr. Gopal Krishan |
Introduction
Utilization of chemical substances, heavy metals in particular, for various day-to-day activities has gradually increased, posing a negative impact on the environment. Anthropogenic activities occurring in varied industrial sectors are the primary source of metal pollution in the environment [1]. Industries require water on a large scale of consumption for different operations, thereby release liquid wastes which in turn enter the aquatic ecosystem through a number of different channels [2,3].
As a major environmental concern for sustainable existence of lives, various technologies in physical, chemical and biological modes have been adapted to diminish and mitigate the noxious effects of these wastewaters. Among the various traditional methods, adsorption is found to be a widely reported feasible method due to its many advantages, such as offering flexibility in design and operation along with regeneration of spent sorbents which add upto the operation's economy [4,5]. A variety of commercial and natural materials have been utilized to treat industrial discharges contaminated with heavy metals and other toxicants [6,7,8]. The current study explores into the potential of Magnolia champaca Barks (MCB), a natural low-cost sorbent, for the effective sequestration of heavy metals at small and large levels.
Zinc is one of the ubiquitous elements that enter the ecosystem through both natural and anthropogenic processes. Wood combustion and waste incineration are examples of natural sources. The majority of zinc produced worldwide is used for industrial processes which include hot-dip galvanising, electro-galvanizing, spraying, painting, and also to protect iron and steel from corrosion. Zinc poisoning symptoms include vomiting, tiredness and dizziness, lack of coordination in the muscles, dermatitis, compromised immune system, and other gastrointestinal diseases [9,10,11].
Another element of interest pertaining to this study is Cadmium, since it is well-known for its toxicity and extensively spent in industries such as batteries, mining, pigments, and alloys. Long-term exposure to cadmium can cause cadmium complexes to build up in the kidney, which can cause renal failure, reduced bone mineralization, lung function issues and is also referred to as a human carcinogen. [12,13,14].
Thus, the main objective of the present study involves in understanding the potentiality of the chosen bio-sorbent for the removal of divalent ions of zinc and cadmium.
Materials and Methods
Collection and preparation of adsorbent
Magnolia champaca a tall tree (fig. 1), of the family Magnoliaceae, is a native to India and found throughout Indo-China, Malaysia, Sumatra, Java, and south-western China. The tree barks were gathered from different locales of Coimbatore, Tamilnadu, India. The stacked materials were cleaned using deionized water in order to get rid of the impurities and then scorched completely. The parched materials were later macerated, minimized by grinding in mixer and separated using varied meshes like 85BSS, 72BSS, 52BSS, 36BSS and 22BSS via Scientific Test Molecular Sieves procured from Jayant Scientific Instruments Company, Mumbai.
 | Figure 1: Magnolia champaca tree.
|
Modification of the sorbent material
The categorized material (Magnolia champaca) was processed with 0.1 N HCl to alter the surface features of the sorbent, thereafter called as Treated Magnolia champaca Barks (TMCB). The treated sorbents of various dimensions were cleansed several times with doubly deionized water to reach a neutral pH value. Only the chemically Treated Magnolia champaca Barks (TMCB) have been exploited for further experiments. Fig 2(a – c) represents the cleaned tree barks; raw sieved material and its treated counterpart (85 BSS).
 | Figure 2: (a). MCB
|
 | Figure 2: (b) Pulverized MCB.
|
 | Figure 2: (c). TMCB
|
Microscopic Analysis
Categorized TMCB was analysed using Optical Microscope (Magnus Microscope CH20ILED) so as to resolve the sizes of the particles. The figured particle sizes fitting to the mesh sizes of 85BSS, 72BSS, 52BSS, 36BSS and 22BSS are 0.18 mm, 0.21 mm, 0.30 mm, 0.42 mm and 0.71 mm respectively. Microscopic examination disclosed the mesoporic nature of TMCB. The microscopic structure of MCB and TMCB (0.18 mm) is depicted in Fig 3 (a) and (b).
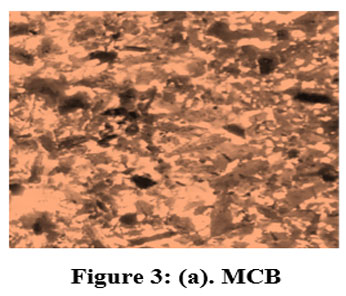 | Figure 3: (a). MCB
|
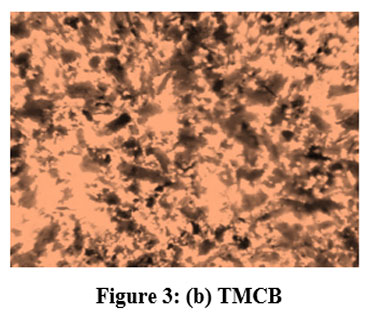 | Figure 3: (b). TMCB
|
Adsorbate (preset solution preparation)
A preset solution of 1000 mg/L Zn (II) / Cd (II) were primed by disbanding appropriate dosage of analytical grade zinc sulphate / cadmium nitrate samples respectively in deionized water. From the preset solution, a standard concentration of 100 mg/L was diluted and subsequent substandard were prepared based on the experimental conditions.
Batch Optimization Studies
Batch experiments were conducted under varied influential parameters viz., particle size (0.18, 0.21, 0.30, 0.42, 0.71 mm); dosage (50 to 300 mg - 50 mg intervals); initial concentration of the sorbate molecules (Zn (II) – 5 to 20 mg/L – 5 mg/L interval; Cd (II) – 2 to 10 mg/L – 2 mg/L interval); agitation time frames (3 to 30 mins- 5 mins intervals); pH (3, 5, 7, 9 and 11) and temperatures, ( 293, 303, 313, 323, 333 K – 10 K interval) so as to optimize the chelating efficiencies of Zn(II) – TMCB / Cd(II) - TMCB systems. 50 mL of the specific divalent standards were added to 250 mL agitating flasks containing appropriate TMCB dosages. These flasks were agitated for preplanned time periods in a mechanical shaker rotating at 140 rpm. The concentrations of the pre and post experimental solutions were analyzed in AAS [Shimadzu AA 6200 Model]. The percentage of sequestrated divalent ions was calculated as follows:
% Removal = (Ci – Ce) / Ci x 100
Characterization Studies
Peak variations relevant to functional groups, surface texture and elemental composition of TMCB and metal laden TMCB were analysed to evidence the metal chelating ability of TMCB. Fourier – Transform Infra-red Spectrophotometer (Shimadzu), Scanning Electron Microscope (SEM) and Energy Dispersive X-ray Analysis (EDAX) were the instruments employed to perform the aforementioned analyses.
Results and Discussion
Fourier Transform Infra-Red Analysis
Fourier Transform Infra-Red spectra of TMCB, its metal loaded counterparts are shown in Fig 4. Disappearing peaks corresponding to hydroxyl groups (3251 cm-1) and alkoxy group (1090 cm-1) in metal laden TMCB spectra suffice the involvement of groups during metal adsorption. A shift in the narrow peak (1629 cm-1) in the unloaded spectra corresponds to C=O stretching. Shift in lower wave numbers (1736 cm-1 and 1794 cm-1) of Zn(II) and Cd(II) loaded spectra evidence their contribution in sorption process. Occurrence of new peaks around 2949 cm-1 and 2937 cm-1 in the post run spectra implies the stretching of C-H bonds in precursors. Notable shifts in peaks less than 900 cm-1 indicates the active changes in C-H bending vibrations, reasoned out due to the binding action of the divalent metals.
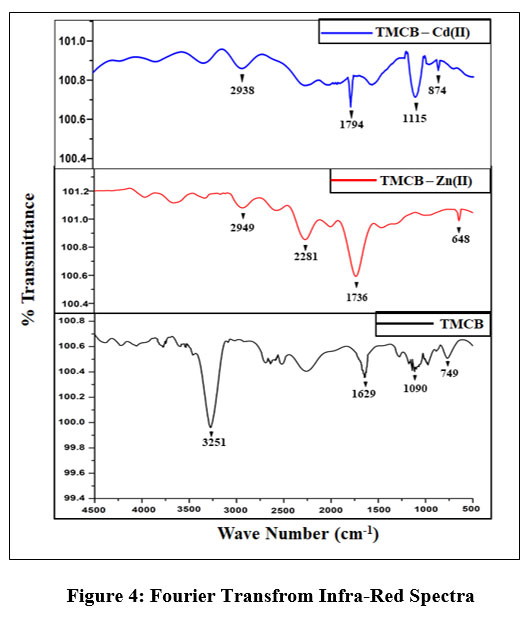 | Figure 4: Fourier Transfrom Infra-Red Spectra.
|
SEM Analysis
SEM micrographs of native and experimentally verified samples are depicted in Fig. 5 (a – c). A highly rugged morphology of Fig 5(a), depicts the presence of open pores aided by acid treatment, which favour the adherence of sorbate molecules onto its surface. However, disappearance of the pores by irregular clusters in figs 5(b) and 5(c) is indicative of metal binding.
 | Figure 5: (a) TMCB
|
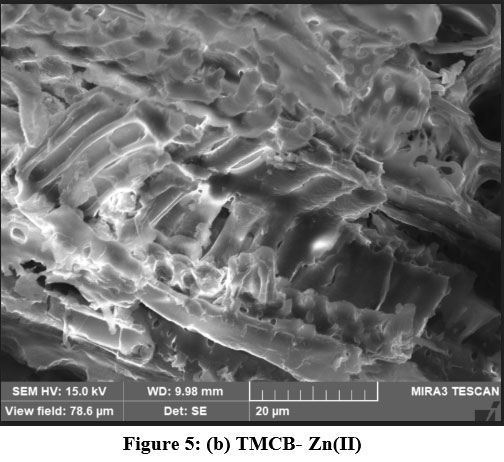 | Figure 5: (b). TMCB-Zn(II)
|
 | Figure 5: (c) TMCB-Cd(II)
|
EDAX Analysis
EDAX spectra were recorded in order to qualitatively examine the presence of elements and their compositions in the unloaded and loaded TMCB. Figures 6(a), (b) and (c) represent the predicted variations in the intensities of the peaks corresponding to Si and appearance of new peaks with respect to Zn and Cd in the range of 2 – 4 keV, correspondingly, which confirms that sorption process had occurred.
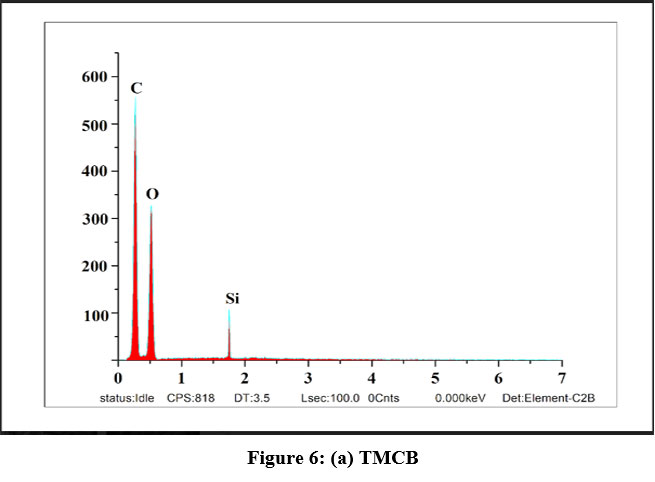 | Figure 6: (a) TMCB
|
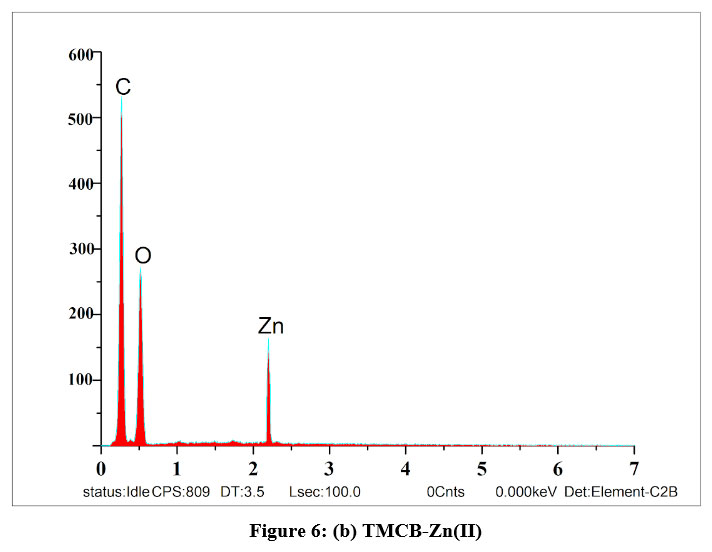 | Figure 6: (b). TMCB-Zn(II)
|
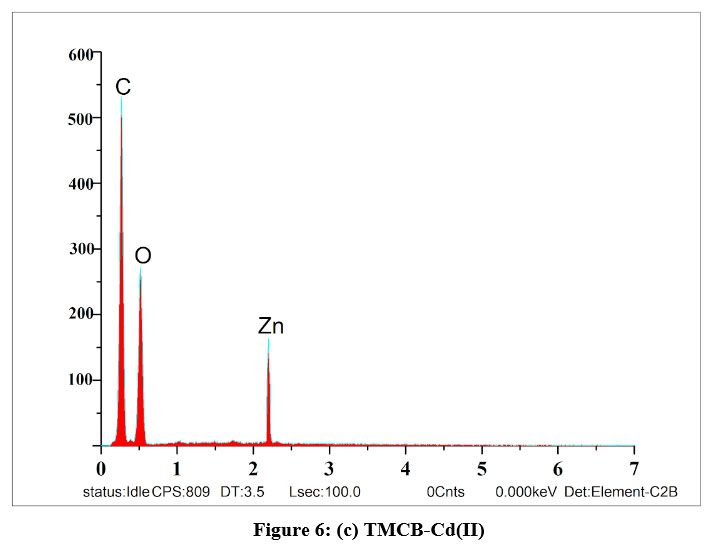 | Figure 6: (c). TMCB-Cd(II)
|
Batch Optimization Studies
Influence of Particle size
Any number of adsorption sites on the surface of a material plays a key role in adsorption efficiency, which is markedly impacted by the particle size. Bar diagram (Fig 7) illustrates the removal of divalent ions by TMCB sorbent of varying particle sizes (0.18, 0.21, 0.30, 0.42 and 0.71 mm). Optimum reduction (as depicted by bar heights) at increasing sample size is evident. A maximum of 98.3% and 96.5% Zn(II) and Cd(II) ions had been sequestrated at the least studied particle size of 0.18mm, supporting the fact, that lower particle size, greater is the surface area and thence higher percentage removal of the sorbate species. Henceforth, 0.18 mm size of TMCB had been chosen for the upcoming experiments.
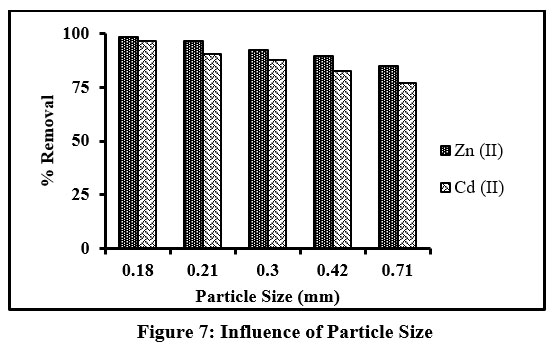 | Figure 7: Influence of Particle Size.
|
Influence of Initial Concentration and Agitation Time
Influence of initial metal ion concentration and agitation time, exhibit an inevitable role in predicting the sorption rate of a system. Fig. 8(a) and (b) elucidates the percentage removal of Zn(II) and Cd(II) ions as 98% and 96% under the optimized conditions of 20 and 10 mg/L, 9 and 12 minutes respectively, within the stipulated experimental particle size, dose and pH conditions.
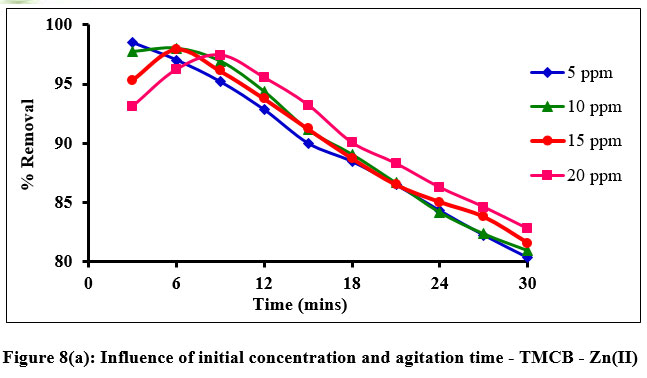 | Figure 8: (a). Influence of initial concentration and agitation time - TMCB - Zn(II).
|
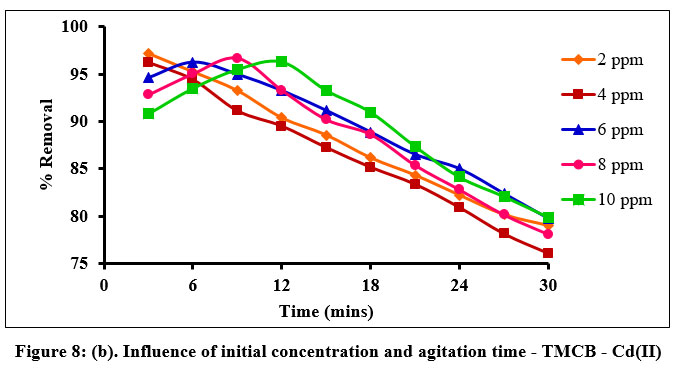 | Figure 8: (b). Influence of initial concentration and agitation time - TMCB - Cd(II)
|
Influence of dosage
Outcomeof the experimental set up, varying the sorbent doses (50 – 300 mg – 50 mg interval) for Zn(II) - TMCB and Cd (II) - TMCB systems is shown (fig. 9). These observations can be justified by the statement that enhanced surface area due to the rise in number of active sites at higher sorbent dosage facilitates easy sorption of metal ions. The peak height in the inverted parabolic curves indicates that major sorption had occurred at 200 mg and 250 mg for Zn(II) and Cd(II) ions, respectively. A decreased sorption had occurred at still higher doses, as indicated by the declined pattern.
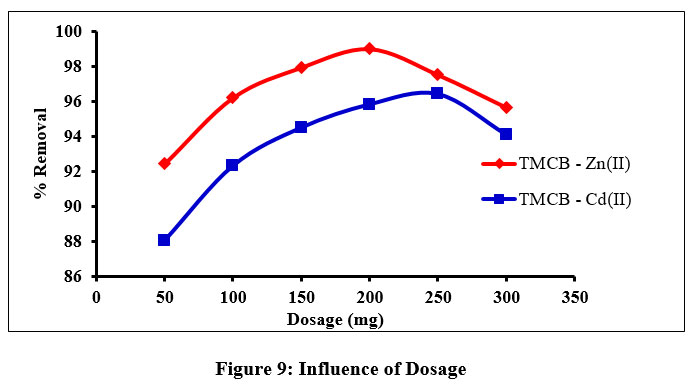 | Figure 9: Influence of Dosage.
|
Influence of pH
pH value of the adsorbate solutions play a vital controlling factor role in any sorbate – sorbent process. In the present study, the initial solution pH values was made acidic as low as 3 and basic as high as 11, by adding 0.1 N HCl and NaOH solutions to the metal solutions of fixed concentrations [20 mg/L: Zn(II) and 10 mg/L: Cd(II)] respectively. The sorbent species registered maximum removal of the studied divalent ions at pH 7 (Fig. 10). The reason for this could be the high protonation of the binding sites by hydronium ions at acidic pH, resulting in repulsion between the sorbent and the sorbate molecules. Further in the basic medium, the metal ion preferentially binds with the hydroxides to form insoluble compounds, thus minimizing the availability of metal cations to get sorbed on TMCB surface.
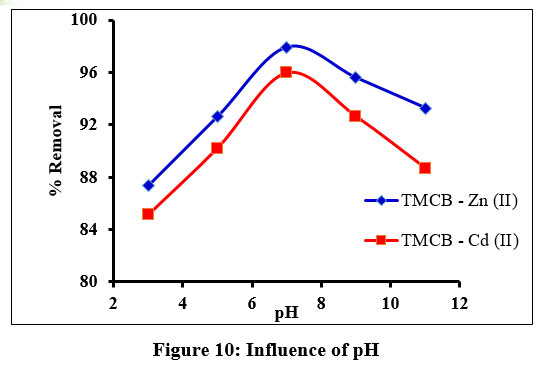 | Figure 10: Influence of pH
|
Influence of Temperature
Of all parameters, significant contribution is noticed by the temperature studies for the adsorption process. Removal of the studied cations under varying temperature agitations is displayed as inverted parabolic curve (fig. 11) with a maximum uptake at 303K / 313 K for Zn(II) / Cd(II) ions. Decreased sorption observed at higher temperatures can be explained by the increased mobility of metal ions at these temperatures.
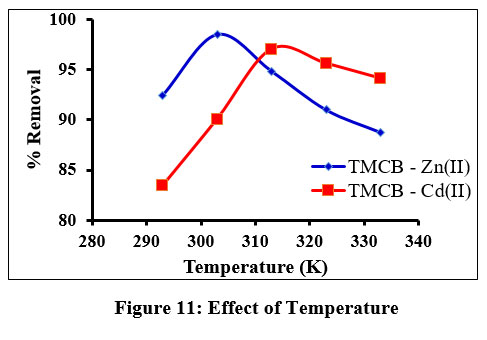 | Figure 11: Effect of Temperature
|
Conclusion
Eco-derived litter material Magnolia champaca Bark was pounded and modified using 0.1 N HCl. The treated powder (TMCB) was employed for chelating Zn(II) and Cd(II) ions. Surface characteristics of treated samples were confirmed by microscopic analysis. Surface morphological changes registered using SEM images supported the statement that sorption had occurred. Involvement of functional groups, morphological variations, peak appearances corresponding to elemental constitution were determined using Fourier Transfrom Infra-Red, SEM & EDAX analyses. Greater metal removal was observed at 0.18 mm particle size at pH 7 for both the analyzed systems, the other parametric factors fixed as 20 mg/l Zn(II), with 9 mins of contact time for dosage of 200 mg TMCB at room temperature; whereas 10 mg/L Cd(II) ions of 12 mins agitation interval for 250 mg sorbent dosage, at a temperature of 313 K. Thus, it is obvious from the made observances that, treated Magnolia champaca Bark is a promising adsorbent for heavy metal contamination of water resources.
Acknowledgement
The authors gratefully acknowledge Tamil Nadu Directorate of Collegiate Education, Chennai, Tamil Nadu, India and the Department of Chemistry, PSGR Krishnammal College for Women, Coimbatore, Tamil Nadu, India.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
The author(s) declares no conflict of interest.
Authors’ Contribution:
All authors contributed to the study conception and design.
Supervision, Formal Analysis, manuscript editing and Plagiarism was done by N. Muthulakshmi Andal
Identification, collection and modification of the material, Batch optimization studies and first draft of the manuscript were done by P. Indhumathy
Data Availability Statement
The manuscript incorporates all datasets produced or examined throughout this research study.
Ethics Approval Statement
All the datas and results pertaining to this current study is the own original work done by the authors in a truthful and complete manner
References
- Crini, G., Lichtfouse, E., Wilson, L.D., Morin-Crini, N., Adsorption-Oriented Processes Using Conventional and Non-conventional Adsorbents for Wastewater Treatment, Green Adsorbents for Pollutant Removal, Envtl. Chem. for a Sus. World, 2018, 18, 23 – 71
CrossRef - Dottoa. G. L., McKay. G, Current scenario and challenges in adsorption for water treatment, J. of Envtl. Chml. Eng.,2020, 8, 1 – 6
CrossRef - Vareda, J. P., Valente, A. J. M., & Durães, L., Assessment of heavy metal pollution from anthropogenic activities and remediation strategies: A review., J. of Envtl. Mngt. 2019, 246, 101–118
CrossRef - Rashid. R, Shafiq. I, Akhter. P, Iqbal M. J., Hussain. M, A state-of-the-art review on wastewater treatment techniques: the effectiveness of adsorption method, Envtl. Sci. and Plln. Res., 2021, 28, 9050–9066
CrossRef - Andal. N. M., and Indhumathy. P, Deployment of toxicants laden sorbents in the manufacture of construction materials – A review, Adv. Appl. Res., 2020,12(1), 43 – 46
CrossRef - Chai. W. S, Cheun. J. Y, Kumar P. S, Mubashir. M, Majeed. Z, Banat. F, Ho. S. H, Show. P. L, A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application, J. of Cln. Prdn. 2021, 296, 1 – 16
CrossRef - Gu. S, Kang. X, Wang. L, Lichtfouse. E, Wang. C, Clay mineral adsorbents for heavy metal removal from wastewater: a review, Envtl. Chem. Lett., 2019, 17, 629–654
CrossRef - Chakraborty, R., Asthana, A., Singh, A. K., Jain, B., and Susan, A. B. H., Adsorption of heavy metal ions by various low-cost adsorbents: a review, Int. J. of Envtl. Analy. Chem., 2022, 102(2), 342 -379
CrossRef - Carolin. C, F., Kumar. P. S., Saravanan. A, Joshiba. G.J., Naushad. M., Efficient Techniques for the Removal of Toxic Heavy Metals from Aquatic Environment: A Review, J. of Envtl. Cheml. Eng., 2017, 5(3), 2782 – 2799
CrossRef - Simon, D., Quaranta, N., Medici, S., Costas, A. and Cristobal, A.S., Immobilization of Zn(II) ions from contaminated biomass using ceramic matrices., J. Hazard. Mater. 2019, 373, 687 - 697
CrossRef - Petcu. C, Purcar. V, Radu. A. L., Ianchis. R, Elvira. A, Sarbu. A, Ebrasud. D. I, Miron. A. R, Modrogan. C, Ciobotaru. A. I., Removal of zinc ions from model wastewater system using bicopolymer membranes with fumed silica, J. Water Process Eng. 2015, 8, 1–10
CrossRef - Rehman. K, Fatima. F, Waheed. I, Akash M. S. H., Prevalence of exposure of heavy metals and their impact on health consequences, J Cell Biochem. 2018, 119, 157–184
CrossRef - Kwikimaa. M. M., Mateso. S, Chebude. Y, Potentials of agricultural wastes as the ultimate alternative adsorbent for cadmium removal from wastewater. A review, Scientific African, 2021, 13, e00934, 1 -14
CrossRef - Bandara. T, Xu. J, Potter. I. D, Franks. A, Chathurika. J. B. A. J, Tang. C, Mechanisms for the removal of Cd(II) and Cu(II) from aqueous solution and mine water by biochars derived from agricultural wastes, Chemosphere, 2020, 254,126745, 1- 10.
CrossRef






