The Protective Role of Vitamin C on Heamatological (WBC and Hb) Alterations in Commom Carp During Chronic Toxicity of Lead Nitrate
1
Department of Life Sciences,
Barkatullah Vishwavidyalaya Bhopal,
India
2
Department of Zoology,
Govt. MVM Bhopal,
India
Corresponding author Email: musratmajeed58@gmail.com
DOI: http://dx.doi.org/10.12944/CWE.18.3.21
Copy the following to cite this article:
Majeed M, Chauhan R, Ahmed T. The Protective Role of Vitamin C on Heamatological (WBC and Hb) Alterations in Commom Carp During Chronic Toxicity of Lead Nitrate. Curr World Environ 2023;18(3). DOI:http://dx.doi.org/10.12944/CWE.18.3.21
Copy the following to cite this URL:
Majeed M, Chauhan R, Ahmed T. The Protective Role of Vitamin C on Heamatological (WBC and Hb) Alterations in Commom Carp During Chronic Toxicity of Lead Nitrate. Curr World Environ 2023;18(3).
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2023-04-04 |
|---|---|
| Accepted: | 2023-12-05 |
| Reviewed by: | 
 Maphuti Kwata
Maphuti Kwata
|
| Second Review by: |

 David Daneesh Massey
David Daneesh Massey
|
| Final Approval by: | Dr. Gopal Krishan |
Introduction
Heavy metal poisoning of local water bodies has resulted from industrial development in developing and developed countries Metal toxicity in aquatic environments has the ability to cause species cellular damage and to disrupt the ecological balance. Elements having relatively high densities, such arsenic, cadmium, mercury, and lead, are potentially hazardous in relatively small quantities referred to as heavy metal). For a healthy metabolism, the human body requires trace levels of a number of heavy metals, including copper, zinc, and selenium. In larger amounts, however, they can cause toxicity. The aquatic environment is an extremely diverse and highly variable system for the continued survival of aquatic species. Occasionally, excessively high amounts of certain metals might be encountered in aquatic life.
The aquatic environment has experienced an increase in the flow of metallic substances because of the massive use of these trace metals has increased throughout the previous several decades Yang and Rose1. Drinking polluted water (from lead pipes, for example), high ambient air concentrations close to emission sources, or ingesting metal ions through the food chain can all result in heavy metal poisoning. This is because the organism takes in heavy metal ions through a process called bioaccumulation, which causes the ions to build up in various organs and tissues. At higher trophic levels, metal concentrations also rise, most likely as a result of amplification of the food chain. Aquatic organisms seem to have metal concentrations that are several orders of magnitude higher than those found in the ecosystem Laws2.
High-density elements that are not biodegradable are known as heavy metals. Due to their lengthy biological half-lives and considerable capacity to accumulate in a variety of bodily entities, they might have adverse effects3,4. The most common metals that can endanger human health at low concentrations are mercury, lead, cadmium, arsenic, and chromium5. Lead and cadmium are highly toxic heavy metals and are an important environmental pollutants, causing intoxication in human and animal tissues and having a great tendency to accumulate in the food chain, causing physiological, biochemical, and neurological dysfunctions in humans6,7,8.
Fish are ideal species to study aquatic ecological systems because of their top positions in the food pyramid and the direct effects that heavy metals have on other animals, including humans.9 Fish exposed to heavy metals may accumulate in various organs, which may subsequently resulted in generating free radicals and leading to demage Additionally, it has the potential to damage the erythrocyte membrane, which might alter other haematological parameters and significantly lower erythrocyte mobility10.. When fish are exposed to heavy metals, they may accumulate in various organs, resulting in the generation of free radicals, leading to oxidative stress and affects the erythrocyte membrane, significantly reducing erythrocyte mobility and altering other haematological parameters10, a substantial drop in Hb, PCV, total protein, and glucose, as well as an increase in creatinine levels in lead-treated rats was discovered by Karamala et al11. Where-as other investigations discovered a rise in liver enzymes12 . Furthermore, lead can cause histological changes such as hepatocyte enlargement, portal space vacuolation, and lymphocytic infiltration. Aquatic toxicity tests are being performed to investigate and analyze the possible toxicological effects of compounds on aquatic organisms because these effects are not necessarily dangerous, one of the key goals of these studies is to detect toxins that may affect aquatic life. Vitamin C is a water-soluble vitamin with innumerable applications, the most important of which is to boost the immune system of aquatic organisms and minimizes the negative effects of stress 12,13,14,15 Furthermore, Animals that consume high antioxidants, such as vitamin C, are protected from damaging free radicals that are produced by regular cellular function and other forms of stress16 to maintain the functional as well as structural integrity of immune cells, several micronutrients' antioxidant properties enhance immunity16,17 and conducts an active role in hydroxylation of proline residues in connective tissue and collagen in vertebrates18,19. Therefore, the utilisation of dietary vitamin C is necessary for the appropriate physiological functions of the fish body20. Leukocytes are regarded as key markers for evaluating the health and immune system of fish because they are very sensitive to environmental changes and respond quickly to undesired elements21,22, while as lymphocytes are thought to be in charge of immunological response and the number of lymphocytes in fish blood is substantially higher than in mammals. Since monocytes and neutrophils are vulnerable to heavy metals, immune-suppression can occur23. Vitamin C is widely regarded as a crucial first-line protective factor that reduces or repairs free radicals by giving one electron, then two protons to produce chemically dehydroascorbic acid.. This is because oxidative damage to lipids and lipoproteins in different cellular compartments and tissues causes an increase in the formation of free radicals, which is prevented by vitamin C, an antioxidant 25,26. Apart from interspecies differences such as age, size, and strain of fish, the ascorbic acid need varies depending on the species of fish. The amount of the Vitamin that is consumed determines how much ascorbic acid has to be added to the diet for proper function27.
Simultaneously, there have been reports that ascorbic acid act as an antioxidant and efficient inhibitor against lead various body organs and blood parameters by co-administration with lead. Vitamin C acts as a detoxifier reduces the toxic impact of heavy metals to provide protection to the cell from abnormalities in their structural features. The purpose of this research was to see if ascorbic acid can help with various haematological parameters after sub-chronic lead exposure.
Materials and Methods
Experimental Fish
Common carp (Cyprinus carpio) is most extensively cultivated fish Modanloo et al 28 belongs to Cyprinidae family Linnaeus (1758). It is a freshwater species that can also be found in brackish water. Cyprinus carpio is a huge exotic minnow species native to Europe and Asia. It is found all across the world and is tough in nature, able to withstand a wide range of environments; yet, the IUCN Red list status has classified common carp as "vulnerable." Cyprinus carpio, a medium-sized freshwater fish measuring 12-18cm and weighing 80-120 gms, was collected from a local fish market and acclimatized under laboratory settings for 15 days.
Methods for determining physicochemical parameters
The physicochemical properties of the test water utilized throughout the experiment. This toxicological investigation was carried out during the summer months, when the temperature averaged 27±2.5°C across all model sets. The pH in all treatment groups was alkaline, with a value of 7.5. The concentration of dissolved oxygen varied between 5 and 6 ppm. Carbon dioxide levels fluctuated between 3-4 ppm, which corresponded to oxygen saturation levels in testing tanks. In all of the testing tanks, the total dissolved solid (TDS) level was around 12 ppm. Alkalinity was measured using phenolphthalein and a methyl orange derivative, and the results were 456 ppm for MO and zero for PHP. The total hardness was approximately 224 ppm, while the calcium hardness was 48 ppm. The chloride concentration of the experimental water was found to be 38 ppm. Nonetheless, all tanks tested negative for lead nitrate.
 | Images 1: (A-E) Is the illustration of experimental set-up
|
Determination of LC50
In the current experiment, the LC50 of lead nitrate was determined, and the substance's impact on common carp mortality was assessed using seven different concentrations of the substance. The mortality rate was computed for lead nitrate concentrations. LC100 of lead nitrate was found to be 60 mg/l. Groups exposed to 30–40 mg/l of lead nitrate showed a 50% death rate. As a result, the fish employed in this experiment, common carp, had an acute 96-hour LC50 value of 35 mg/l (ppm).
Experimental design
Fishes were divided into various concentrations of lead nitrate (5, 10, 15, 20 ppm) and the other group received lead nitrate concentration with 450 mg/l of Vitamin C. Fishes that did not respond to tactile stimulation or show any respiratory activity during the experiment were deemed dead and removed right away, and at the conclusion of the exposure time, the experimental species were taken out and blood samples were drawn from the caudal vein
Statistical Analysis
At 24, 48, 72, and 96 hours, the fish mortality in the various lead nitrate concentrations was noted. The acquired values were transformed into log concentrations for statistical analysis, and probit analysis was used to determine the mortality percentage (Finney, 1971)29.Every result collected is shown as mean ± standard deviation. The heavy metals-treated groups and the control group underwent one-way analysis of variance (ANOVA), which was then conducted using SPSS.
Results
Haematological changes associated with Lead nitrate.
Blood analysis should be the most conclusive instrument for determining an animal's health status. In the case of fish, blood is the best sign of stress, as evidenced by changes in parameters. During the current study period, common carp fingerlings were tested for RBC, haemoglobin, PCV, MCV, MCH, and MCHC after being subjected to various sub-lethal amounts of lead nitrate. Of course, heavy metals will alter blood parameters based on the concentration of heavy metals present and at time of exposure. The aim of the experiment is to look at the preventive part of Vitamin C in maintaining homeostasis and reversing the effects of exposure. During the current study, all test groups were subjected to various amounts of lead nitrate (5, 10, 15, 20 ppm) alone and in combination with lead nitrate and 450 mg/l Vitamin C. Many researchers have identified Vitamin C's protective role, including30,31. Sub-lethal amounts of lead nitrates were shown to significantly alter haematological values in common carp, but Vitamin C was found to have an important role in stabilizing haematological parameters and thus the health of the fish. The outputs of the current research term have resulted in several critical outcomes that may be required for monitoring the fish's prime health conditions.
Effects of lead nitrate on (White Blood Corpuscles (WBC’s
The variations in WBC content in Common carp exposed to various concentrations of Lead nitrate 5, 10, 15, 20ppm for the period of 15, 30, 60 and 90 days respectively, in exposed groups tend to be protected with 450 mg/l Vitamin C
Control group
The mean±SD value of WBC (White Blood Corpuscles) expressed in x 103/µL was 4645±210.2 in control group.
Treated group: The fishes exposed to 5.0 parts per million of lead nitrate for 15 days. (16.6% of 96 h LC50) Throughout a 15-day period revealed no discernible (P>0.005) increase in WBC count 4645± 156.5 (0.00), which increased by a considerate coefficient in the same test group protected with Vitamin C (4651± 156.2 (l0.12)), recording zero covariance between the groups.
The fishes exposed to 30 days at 5.0 parts per million of lead nitrate: Similar trend was recorded in 30 days PE group with WBC count of 4755± 198.2 (l2.36).
The fishes exposed to 5.0 parts per million of lead nitrate for 60 days. There was significant increase in WBC count in test group set exposed for a duration of 60 days (4788± 210.2 (l3.07)), recording a covariance of 10224.5 between the groups. Significant (P<0.001) changes were observed in 30 days group with a covariance of 6050.
The fishes exposed to Lead nitrate at 5.0 parts per million for 90 days Post 90 days exposure, a significant (P<0.001) decrease was observed in WBC count (4685± 119.5 (0.86)) in fishes protected with ascorbic acid, recording the role of vitamin C in restoring homeostasis in fishes exposed to lead nitrate.
Generally, as the dose and duration of lead exposure increased, the white blood cell count increased and the count decreased very meagerly in test fishes protected by vitamin C, however, the duration showed a negative impact on the white cell count. The graphical demonstration of changes between the exposure groups based on duration are presented in figure 1.
.jpg) | Figure 1: Correlative change in WBC count via linear trend line between the test groups and visual impact of duration on the fluctuations in white cell count.
|
In test set exposed to 10 ppm for period of 15 days
The fishes showed an increase in value of WBC (4702±110.2 (l1.22)) in first 15 days of exposure, which got drastically increased to (4828±200.4 (l3.93)) in fishes exposed over a period of 90 days. However significant (P<0.005) decrease was reported in WBC count in 15 days PE group protected with vitamin C (4670±156.3 (l0.53)), showing a covariance of 1624.5 between the groups. A significant (P<0.001) decrease was reported in WBC count in 90 days PE Vitamin C protected group (4722± 165.3 (l1.65)), as compared with the Vitamin C free group, recording a covariance of 16744.5.
In test set exposed to 10 ppm for the period of 90 days
(33.0% of 96 h LC50) lead nitrate over duration of 90 days showed remarkable fluctuations in WBC count about the amount and length of exposure. More or less similar changes were reported in fishes exposed to 15 ppm (50.0% of 96 h LC50) lead nitrate over duration of 90 days exhibited significant variations in WBC count .The fishes showed an increase in value of WBC (4701±125.6 (l1.20)) in first 15 days of exposure, which got drastically increased to (4872±154.2 (l4.88)) in fishes exposed over a period of 90 days. However significant (P<0.005) decrease was reported in WBC count in 15 days PE group protected with vitamin C (4785±144.2 (l3.01)), showing a covariance of 25764.5 between the groups.
In the experimental set of fishes exposed to 20 ppm
(66.6% of 96 h LC50) lead nitrate over duration of 90 days exhibited significant variations in WBC count in cooperation with the quantity and duration of exposure. Fish demonstrated that there had been a notable rise (P<0.001, 0.01) in value of WBC (4774±165.7 (l2.77)) in first 15 days of exposure, which got drastically increased to (4908±201.3 (l5.66)) in fishes exposed over a period of 90 days. However significant (P<0.001) decrease was reported in WBC count in 15 days PE group protected with vitamin C (4711±201.1 (l1.42)), showing a covariance of 4900.5 between the groups. A significant (P<0.005) decrease was reported in WBC count in 90 days PE Vitamin C protected group (4795±156.8 (l3.22)), as compared with the Vitamin C free group, recording a covariance of 35485.5
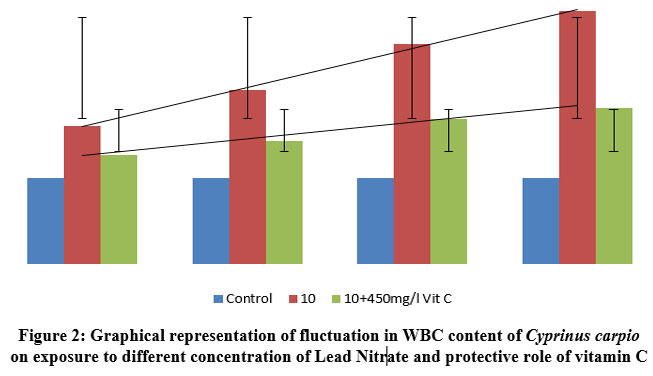 | Figure 2: Graphical representation of fluctuation in WBC content of Cyprinus carpio on exposure to different concentration of Lead Nitrate and protective role of vitamin C
|
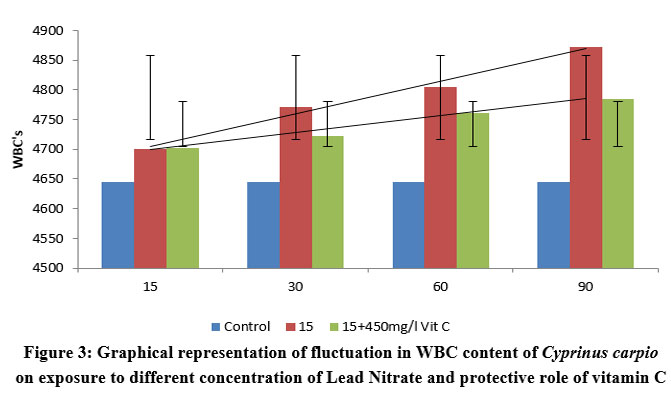 | Figure 3: Graphical representation of fluctuation in WBC content of Cyprinus carpio on exposure to different concentration of Lead Nitrate and protective role of vitamin C
|
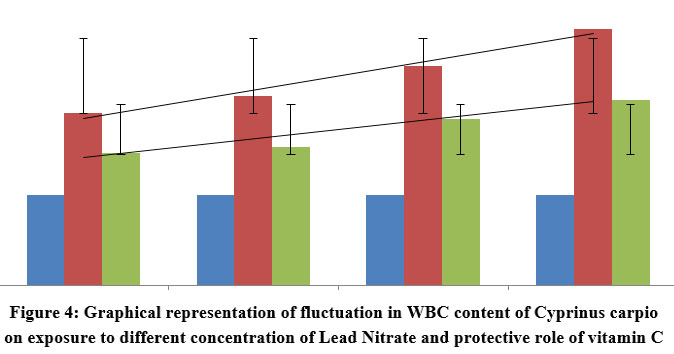 | Figure 4: Graphical representation of fluctuation in WBC content of Cyprinus carpio on exposure to different concentration of Lead Nitrate and protective role of vitamin C
|
As immune cells, WBCs increased in response to increased lead nitrate exposure duration and time. This is an example of the protective behaviour that WBCs exhibit in fish bodies. However, there was less increase in WBC’s in Ascorbic acid protected groups as compared to the control species, but showed a great percentile of recovery in Ascorbate protected group as compared to the test group, contesting the ability of the vitamin to cope up with the stress. The graphical representation showing the linearty in changes in WBC is depicted in figure 2. One way ANOVA between the WBC count in fishes exposed to various concentrations of lead nitrate revealed Significant (P<0.0001) changes as depicted in table 3. Similar results were obtained with significant (P<0.0001) changes in vitamin C protected groups.
Table 1 Statistical interpretation in WBC’s (X 103/µL) of Cyprinus carpio with respect to exposure of various concentrations of Lead Nitrate and Vitamin C as Protector
Lead Nitrate Conc. PPM | Control | Duration of Exposure | |||
15 days PE | 30 days PE | 60 days PE | 90 days PE | ||
5.0 | 4645±210.2 | 4645±156.5(0.00)ab | 4755±198.2(I2.31)ac | 4788±210.2(I2.98)abc | 4800±300.1(I3.22)abd |
5.0 with Vit C 450mg/l | 4651±156.2(I0.12)acd | 4662±144.5(I1.99)acd | 4671±120.0(I2.50)ab | 4685±119.5(I2.45)acd | |
CoVar | 0.00 | 6050 | 10224.5 | 12012.5 | |
10.0 | 4702±110.2(I1.21)ad | 4741±201.2(I2.02)ac | 4792±210.2(I3.06)ad | 4828±200.4(I3.79)ac | |
10.0 with Vit C 450mg/l | 4670±156.3(I0.68)ad | 4685±185.2(I1.19)ab | 4710±145.6(I1.74)ac | 4722±165.3(I2.24)acd | |
CoVar | 1624.5 | 4608 | 10804.5 | 16744.5 | |
15.0 | 4701±125.6(I1.19)acd | 4771±184.6(I2.64)ac | 4804±165.4(I3.30)abc | 4872±154.2(I4.65)acd | |
15.0 with Vit C 450mg/l | 4702±165.3(I0.02)bc | 4722±148.2(I1.03)ac | 4761±165.9(I0.90)cd | 4785±144.2(I1.81)b | |
CoVar | 1568 | 7938 | 12640.5 | 25764.5 | |
20.0 | 4774±165.7(I2.70)cd | 4801±155.2(I3.24)ac | 4848±164.8(I4.18)acd | 4908±201.3(I5.35)ac | |
20.0 with Vit C 450mg/l | 4711±201.1(I1.33)ad | 4721±200.0(I1.69)bc | 4765±69.5(I1.74)ac | 4795±156.8(I2.35)ac | |
CoVar | 4900.5 | 12168 | 20604.5 | 35485.5 | |
(i): Percent depression; (I): Percent Stimulation
a (P<0.01), b(0.05), c(0.005), d(0.005) Values with no common superscript differ significantly
Table 2. One Way ANOVA for Lead Nitrate concentration vs WBC in Cyprinus carpio
Anova: One Way | ||||||
SUMMARY | Count | Sum | Average | Variance |
|
|
5 | 4 | 70 | 17.5 | 41.66667 |
|
|
10 | 4 | 19032 | 4758 | 5350 |
|
|
15 | 4 | 19215 | 4803.75 | 4890.25 |
|
|
20 | 4 | 19429 | 4857.25 | 7832.917 |
|
|
|
|
|
|
|
|
|
ANOVA | ||||||
Source of Variation | SS | Df | MS | F | P-value | F crit |
Rows | 74208850 | 4 | 18552212 | 3731.313 | 2.77E-22 | 3.055568 |
Columns | 74580.5 | 15 | 4972.033 |
|
|
|
Total | 74283430 | 19 |
|
|
|
|
Table 3: One Way ANOVA for Lead Nitrate concentration + Vit C vs WBC in Cyprinus carpio
Anova: One Way | ||||||
SUMMARY | Count | Sum | Average | Variance |
|
|
5 | 4 | 70 | 17.5 | 41.66667 |
|
|
10 | 4 | 18820 | 4705 | 764.6667 |
|
|
15 | 4 | 18910 | 4727.5 | 1616.333 |
|
|
20 | 4 | 19046 | 4761.5 | 1672.333 |
|
|
|
|
|
|
|
|
|
ANOVA | ||||||
Source of Variation | SS | Df | MS | F | P-value | F crit |
Rows | 71499575 | 4 | 17874894 | 14969.14 | 8.31E-27 | 3.055568 |
Columns | 17911.75 | 15 | 1194.117 |
|
|
|
Total | 71517487 | 19 |
|
|
|
|
Effects of lead nitrate on (Hemoglobin (g/dL) of common carp (C. carpio)
Based on the studies conducted results showed in table 5. The mean±SD value of HB (Haemoglobin) expressed in g/dl was 8.20±0.28 in control group. The fishes exposed to 5.0 ppm Lead nitrate (16.6% of 96 h LC50), PE demonstrated a substantial (P<0.01, 0.001) decline throughout the first 15 days (1.82%) in Hb concentration (8.05± 0.26 (I1.82)), in contrast to the group under control, which decreased considerably (P<0.01, 0.005) by 3.41% in vitamin C treated group, reporting a covariance of 0.011 (7.92± 0.11 (I3.41). In 30 days PE group, the Hb concentration showed a significant (P<0.05, 0.005) decrease to a value of 7.89± 0.44 (I3.78) compared to the group that is controlled, which further got significantly (P<0.05, 0.005) reduced to the value of 7.40± 0.35 (I9.75) 90 days PE. However, in vitamin C treated group, a significant (P<0.05, 0.005, 0.001) increase (7.92%) was observed (7.55± 0.32 (I7.92)) after 90 days PE, recording a covariance of 0.320 between the groups. Generally, as the dose and duration of lead exposure in test fishes increased, the haemoglobin concentration in Common carp decreased drastically, as compared to the vitamin C protected group, which clearly depicts the protective role of vitamin C.
Statistical interpretation in Hb (g/dL) of Cyprinus carpio with respect to exposure of various concentrations of Lead Nitrate and Vitamin C as Protector
Table 4
Lead Nitrate Conc. PPM | Control | Duration of Exposure | |||
15 days PE | 30 days PE | 60 days PE | 90 days PE | ||
5.0 | 8.20±0.28 | 8.05±0.26(I1.86)b,d | 7.89±0.44(l3.92) a,c | 7.48±0.22(l9.62) a,b | 7.40±0.35(l10.8) a,c |
5.0 with Vit C 450mg/l | 7.92±0.11(I1.64) b,c | 7.90±0.24(l0.12)b,d | 7.62±0.21(l1.83)a,c | 7.55±0.32(l1.98)a,cd | |
CoVar | 0.011 | 0.048 | 0.259 | 0.320 | |
10.0 | 7.85±0.47(l4.45)abc | 7.55±0.38(l8.60) a,d | 7.40±0.32(l10.8) a,c | 7.20±0.31(l13.8)acd | |
10.0 with Vit C 450mg/l | 7.90±0.32(l0.63) a,c | 7.65±0.32(l1.30) b,c | 7.45±0.24(l0.67) acd | 7.40±0.28(l2.70) ac | |
CoVar | 0.0612 | 0.211 | 0.32 | 0.5 | |
15.0 | 7.65±0.39(l7.18) abc | 7.36±0.56(l11.4) ac | 7.20±0.28(l13.8)bc | 6.90±0.24(l18.8)ac | |
15.0 with Vit C 450mg/l | 7.60±0.32(l0.40)cd | 7.50±0.33(l1.67) ac | 7.22±0.51(l2.03) a,d | 6.90±0.26(l2.98) a,d | |
CoVar | 0.1512 | 0.3528 | 0.50 | 0.845 | |
20.0 | 7.42±0.42(l10.5)bc | 7.03±0.11(l16.6)bc | 6.75±0.22(l21.4)ad | 6.50±0.23(l26.1) bc | |
20.0 with Vit C 450mg/l | 7.45±0.24(l0.40)ac | 7.15±0.21(l1.67) a,c | 6.89±0.12(l2.03) a,d | 6.70±0.11(l2.98)abd | |
CoVar | 0.3042 | 0.684 | 1.051 | 1.445 | |
(l): Percent depression; (l): Percent Stimulation
a (P<0.01), b(0.05), c(0.005), d(0.005) Values with no common superscript differ significantly
Table 5. One Way ANOVA for Lead Nitrate concentration vs Hb in Cyprinus carpio
Anova: One Way | ||||||
SUMMARY | Count | Sum | Average | Variance |
|
|
5 | 4 | 70 | 17.5 | 41.66667 |
|
|
10 | 4 | 29.95 | 7.4875 | 0.140625 |
|
|
15 | 4 | 29.16 | 7.29 | 0.126067 |
|
|
20 | 4 | 28.21 | 7.0525 | 0.124825 |
|
|
|
|
|
|
|
|
|
ANOVA | ||||||
Source of Variation | SS | Df | MS | F | P-value | F crit |
Rows | 341.9189 | 4 | 85.47973 | 10.12617 | 0.000354 | 3.055568 |
Columns | 126.6221 | 15 | 8.44147 |
|
|
|
Total | 468.541 | 19 |
|
|
|
|
Table 6. One Way ANOVA for Lead Nitrate concentration +Vit C vs Hb in Cyprinus carpio
Anova: One Way | ||||||
SUMMARY | Count | Sum | Average | Variance |
|
|
5 | 4 | 70 | 17.5 | 41.66667 |
|
|
10 | 4 | 12.53 | 3.1325 | 0.003892 |
|
|
15 | 4 | 12.04 | 3.01 | 0.025133 |
|
|
20 | 4 | 11.71 | 2.9275 | 0.029892 |
|
|
|
|
|
|
|
|
|
ANOVA | ||||||
Source of Variation | SS | Df | MS | F | P-value | F crit |
Rows | 677.1051 | 4 | 169.2763 | 20.2554 | 6.58E-06 | 3.055568 |
Columns | 125.3564 | 15 | 8.357095 |
|
|
|
Total | 802.4615 | 19 |
|
|
|
|
In fishes exposed to 10 ppm (33.0% of 96 h LC50) lead nitrate, the fishes revealed a significant (P<0.05, 0.01, 0.005) decline in value of Hb concentration (7.85±0.47 (l4.26)) during first 15 days of exposure, which got significantly (P<0.05, 0.005) reduced to (7.20±0.31 (l12.2)) in fishes exposed over a period of 90 days. No significant differences were reported in 15 days PE groups, with a significant (P<0.05, 0.005) elevation in vitamin C protected group (7.90±0.32 (l3.65)), as compared to the test group, showing a covariance of 0.0612. As the exposure period extended to 90 days, A noteworthy (P<0.05, 0.01, 0.001) decrease was seen in haemoglobin concentration (7.20± 0.31 (l12.2)), compared to the control group of species, which had a significant increase (P<0.05, 0.005) by a margin of 9.75% in test group protected with vitamin C post 90 days exposure (7.40±0.28 (l9.75)), recording a covariance of 0.5.
In 15 ppm (50.0% of 96 h LC50) exposure trial, the test fishes exhibit a notable (P<0.05, 0.01, 0.005) reduction in value of Hb (7.65±0.39 (l6.70)) during first 15 days of exposure, which got significantly (P<0.05, 0.005) reduced to (6.90±0.24 (l15.8)) in fishes exposed over a period of 90 days. However, significant (P<0.005, 0.001) increase was reported in Hb concentration in 15 days PE group protected with vitamin C (7.60±0.32 (l7.31)), showing a covariance of 0.1512 between the groups. No remarkable increase was reported in Hb concentration in 90 days PE Vitamin C protected group (6.90± 0.26 (l15.8)), as compared with the test group, recording a covariance of 0.32, which could be as a result of some other stressors during the experimental tenure.
The fishes exposed to 20 ppm (66.6% of 96 h LC50) lead nitrate over duration of 90 days exhibited significant variations in Hb concentration. The fishes showed a remarkable (P<0.01, 0.005) decline in value of Hb (7.42±0.42 (l9.51)) during first 15 days of exposure, which got significantly (P<0.05, 0.005) increased to (7.45±0.24 (l9.14)), in vitamin C protected group, compared to the experimental group. A significant (P<0.05, 0.01, 0.001) increase (18.3%) was reported in Hb concentration in 90 days PE Vitamin C protected group (6.70±0.11 (l18.3)), as compared to the test group, recording a covariance of 1.445, One way ANOVA between lead nitrate concentrations (Table 4) above mentioned and lead nitrate with vitamin as a protectant (Table 5) revealed significant (P<0.0001) results.
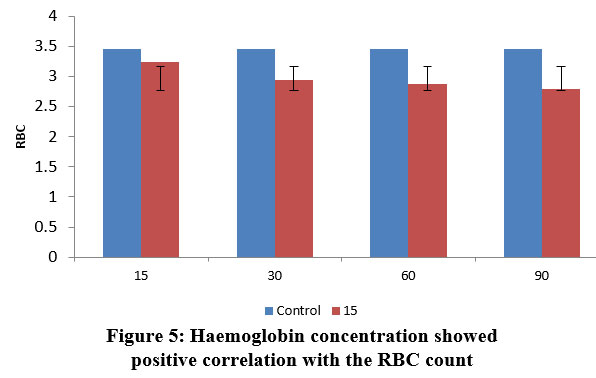 | Figure 5: Haemoglobin concentration showed positive correlation with the RBC count.
|
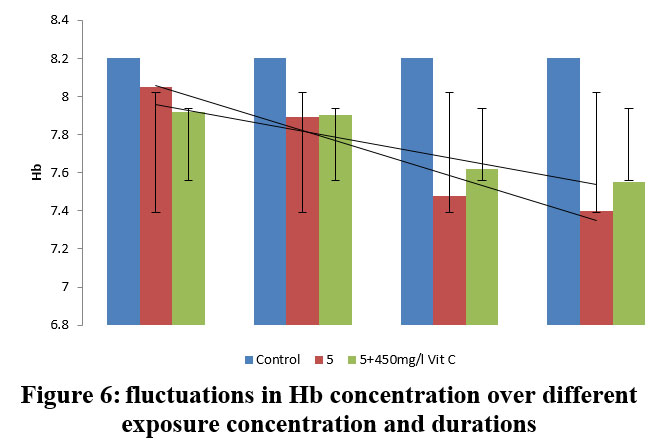 | Figure 6: fluctuations in Hb concentration over different exposure concentration and durations.
|
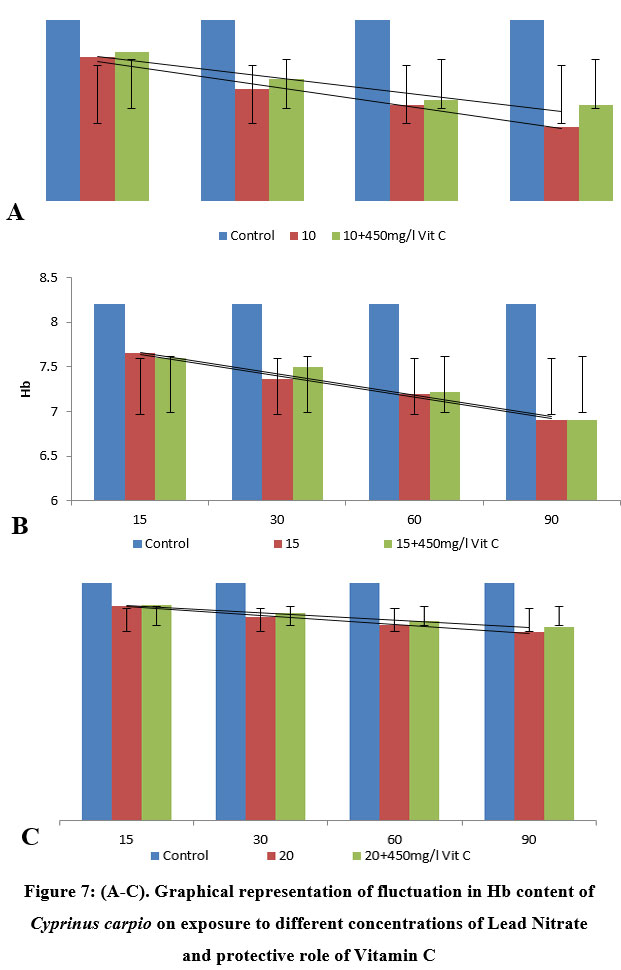 | Figure 7: (A-C): Graphical representation of fluctuation in Hb content of Cyprinus carpio on exposure to different concentrations of Lead Nitrate and protective role of Vitamin C
|
Haemoglobin level fluctuations can potentially cause havoc with the entire circulatory system. Because of stress caused on by fluctuating lead nitrate concentrations, the fish were unable to supply adequate oxygen to the blood tissues, which led in this noticeable fluctuation in haemoglobin concentration.
Discussion
Hematological evaluations serve as tools for assessing the health state of fish and indicate the detrimental impact of nanoparticles or the protective effects of nutritional supplements32 .The current study found that the exposure of lead nitrate to Cyprinus carpio increased with the duration and time of lead nitrate exposure, which is a protective behaviour demonstrated by the WBCs in the fish body. However, there was a lower increase in WBCs in the Vitamin C protected group compared to the control group, but a higher percentile of recovery in the Vitamin C protected group correlated to the test group, calling into question the vitamin's ability to cope with stress. The connection between fish nutrition and welfare is a crucial component of aquaculture. Fish's inadequate nutritional requirements lead to higher mortality rates, which necessitates the development of artificial diets that are suitable Erythropoiesis, hemosynthesis, osmoregulatory dysfunction,for intensive production systems33,these anti-oxidants protect tissues from free radicals' toxic effects34
The repercussions suggest that vitamin C treatment alleviates lead-induced changes to some extent; the results also show a considerable shift in the peroxidative process. When exposed to lead-treated groups, researchers notice a considerable drop in haematological profile WBC, RBC, and Hb (FIG), establishing an erythropenia and hemolysis status in C. batrachus35. Hemosynthesis, osmoregulatory dysregulation, erythropoiesis, erythrocyte breakdown of hematopoietic system are possible explanations for the reduction in RBC and Hb. The liver portions in the vitamin C supplementation group at 200 mg/kg revealed normal, healthy liver histology with clogged sinusoids, while the Sections of the liver taken from the 300 mg/kg vitamin C group revealed some Kupfer cell hyperplasia but otherwise normal hepatic tissues. The group that received 400 mg/kg of vitamin C had normal hepatic tissues and a few fatty vacuoles36. In accordance to our experimental period ascorbic acid at 450 mg/kg when administered to fish, the haemoglobin count decreases less, than it does in fish that have been exposed to heavy metals. Fish pre-exposed to lead nitrate recovered fast in terms of haemoglobin count in ascorbic acid when compared to a natural treatment in normal water. According to the current study investigation that lead and cadmium frequently found in the environment, particularly in food haematological and biochemical changes. Pre-administration of antioxidants and heavy metals (lead and cadmium) has a protective impact on haematological and biochemical change additionally, it may be concluded from current research that vitamin C has strong antioxidant effect against lead and cadmium intoxication. It is highly suggested to consume foods high in vitamin C to lessen the harm brought on by lead exposure37. Due to the metal's direct impact on haematological architecture, inappropriate Fe metabolism, and/or decreased intestinal absorption of Fe as a result of mucosal lesions in Anguilla rostrata38, a reduction in haemoglobin after chronic cadmium exposure has been associated to decreased erythropoiesis, fish exposed to pesticides have lower haemoglobin counts, which is due to haemoglobin disruption, which also prevents aerobic glycolysis.
Conclusion
WBC’s being the immune cells showed an increase with the increase in the duration and exposure time of lead nitrate, which is a protective behaviour shown by the WBC’s in fish body. However, there was less increase in WBC’s in vitamin C protected group, as compared to the untreated group, but showed a great percentile of In comparison to the experimental group, the vitamin C maintained group demonstrated enhanced recovery contesting the ability of the vitamin to subsist with the stress and functions as a detoxifier, reducing pesticide toxicity and protecting the cell from structural anomalies. The haemoglobin concentration in common carp decreased significantly when test fish were exposed to higher doses and longer durations of lead, in contrast to the vitamin C protected group, indicating the protective effect of vitamin C.
Acknowledgement
The authors would like to thank HOD Department of zoology, Govt. MVM Bhopal, for assisting and cooperating with us for providing laboratory facilities for the whole research.
Conflict of Interest
I confirm that there are no conflicts of interest in this piece of content.
Funding Sources
The authors received no financial/funding support for the research or publication of this article.
References
- Yang H and Rose N.L. Distribution of Hg in the lake sediments across the UK. Sci. Total Environ.2003; 304: 391-404.
CrossRef - Laws E.A. Aquatic Pollution- An introductory text. John Wiley and Sons. 2000; New York, U.S.A. 309-430.
- Sathawawara N.G, Parikh D.J, Y.K. Agarwal. Essential Heavy metals in Environmental samples from Western India. Ball. Environ. Contam. Toxicol 2004 73: 756-761.
CrossRef - Bagdatlioglu N, C. Nergiz and Ergonul P.G. Heavy Metal Levels in Leafy Vegetables and some Selected Fruits. Journal of Consumer Protection and Food Safety 2010 5: 421-428.
CrossRef - Al-fatlawi A.C, Al-Murshedi M.H. The Effects of Heavy Metal (Nickel) on Hematological Parameters of Laboratory Male Mice. International Journal of Advanced Research. 2015; 3(9): 598- 601.
- Flora S.J.S, Flora G. and Saxena G. Environmental Occurrence, Health Effects and Management of Lead Poisoning. In: Jose SC and Jose S, editors. Lead. Amsterdam. Elsevier Sci. 2006; 158-228.
CrossRef - Hounkpatin, A.S.Y, R.C. Johnson, P. Guedenon, E. Domingo, C.G. Alimba, M. Boko and P.A. Edorth. Protective effects of vitamin C on hematological parameters in intoxicated Wister rats with cadmium, mercury and combined cadmium and mercury. International Research Journal of Biological Sciences. 2012; 1(8): 76-81.
- Deveci. E. Ultrastructural. Effects of Lead Acetate on Brain of Rats. Toxicol. Ind. Health. 2006; 22(10): 419-422.
CrossRef - Van der Oost, R. Beyar, J, Vermeulen, N.P.E. Fish bioaccumulation and biomarkers in environmental risk assessment: A review. Environmental Toxicology and Pharmacology. 2003; 13: 57-149.
CrossRef - Gills. S, H. Tewari, J. Ponde. Effect of water borne copper and lead on the Peripheral blood in the Rosy Barb, barbus. Bull Environ Contam. Toxicol. 2009; 46: 606-612.
CrossRef - Karamala, S.K, S. Latha, Y. Anjaneyulu, T.S. Chandra, V.D. Sreeni, A.P. Pidugu. Hematobiochemical changes of lead poisoning and amelioration with Ocimum sanctum in wistar albino rats. Veterinary World. 2011; 4(6): 260-263.
CrossRef - Hardie, L.J, Fletcher. T.C, Secombs, C.J. The effect of dietary vitamin C on the immune response of Atlantic salmon (Salmo salar L.). Aquaculture,1991. 95: 201 214. Head, K.A.(1998). Ascorbic acid in the prevention and treatment of cancer. Altern Med Rev 3(3): 174-186.
CrossRef - Henrique, M.M.F, Gomes.E.F, Gouillou-Coustans, M.F, Oliva-Teles. A, Davies, S.J. Influence of supplementation of practical diets with vitamin C on growth and response to hypoxic stress of seabream, Sparus aurata. Aquaculture. 1998; 161: 415–426
CrossRef - Petric, M.C, Martins, M.L Onaka, E.M, Moraes, J.R.E, Moraes, F.R, Malheiros, E.B. Dietary vitamin C supplementation potentiates the formation of giant cells in pacu Piaractus mesopotamicus Holmberg, 1887 (Osteichthyes: Characidae). Boletim do Instituto de Pesca. 2003; 29: 69- 76.
- Sarma. K, Pal.K. Sahu, P, Ayyappan, S. and Baruah, K. Dietary high protein and vitamin C mitigates endosulfan toxicity in the spotted murrel, Channa punctatus, Sci. Total Environ. 2009; 407: 3668–3673. Bloch
CrossRef - Chew, B. P. (1995). Antioxidant vitamins affect food animal immunity and health. J. Nutri.125: 18045-18085.
- Innocent, B.X, Fathima, M.S.A, Sivagurunathan. A. Haematology of Cirrhinus mrigala fed with Vitamin C supplemented diet and post challenged by Aphanomyces invadens. Journal of Applied Pharmaceutical Science 01 (09) 2011; 141- 144.
- Wang, X; Kim, K; Bai, S.C; Huh, M. and Cho, B. Effects of the different levels of dietary vitamin C on growth and tissue ascorbic acid changes in parrot fish (Oplegnathus fasciatus). Aquaculture. 2003; 215: 203–211.
CrossRef - Zhou. X, Xie, M. Niu, C, Sun, R. The effects of dietary vitamin C on growth, liver vitamin C and serum cortisol in stressed and unstressed juvenile soft shelled turtles (Pelodiscus sinensis). Comp. Biochem. Physiol. Mol. Integr. Physiol. 2003;135: 263– 270.
CrossRef - Zou, W Lin, Z. Huang, Y. Limbu, S.M. Rong, H. Yu, C. Lin, F, Wen, X .Effect of dietary vitamin C on growth performance, body composition and biochemical parameters of juvenile Chu's croaker (Nibea coibor). Aquaculture Nutrition. 2020; 26:60–73.
CrossRef - Hughes, G.M. and Nemcsok, J. Effects of low pH alone and combined with copper sulphate on blood parameters of rainbow trout. Environ. Pollut. 1988; 55: 89-95.
CrossRef - Tavares-Dias, M. and Moraes, F.R. Haematological parameters for the Brycon orbignyanus Valenciennes, (Osteichthyes: Characidae) intensively bred. Hidrobiologica .2006; 16: 271-274.
- Witeska, M. and Wakulska, M. The effects of heavy metals on common carp white blood cells in vitro. Alternatives to Laboratory Animals, Baltimore. 2007; 35: 87-92.
CrossRef - Gupta, R.S., E.S. Gupta, B.K. Dhakal, A.R. Thakur , J. Ahnn. Vitamin C and vitamin E protect the rat testes from cadmium-induced reactive oxygen species. Mol. Cells.2004; 17: 132-139
- Carr. A, B. Zhu, B. Frei, "Potential Antiatherogenic Mechanisms of Ascorbate and Alphatocopherol" Circulation Research. 2000; 87: 349-354.
CrossRef - Halliwell, B.Role of free radicals in the neurodegenerative diseases: Therapeutic implications for antioxidant treatment" Drugs Ageing. 2001; 18(9): 685-716.
CrossRef - Lim, C. and Webster, C.D. Nutrition and fish health. The Haworth press. 2001; 163. New York, U.S.A.
CrossRef - Modanloo, M; Soltanian, S; Akhlaghi, M. and Hoseinifar, S.H. The effects of single or combined administration of galactooligosaccharide and Pediococcus acidilactici on cutaneous mucus immune parameters, humoral immune responses and immunerelated genes expression in common carp (Cyprinus carpio) fingerlings. Fish Shellfish Immunol. 2017; 70:391–397.
CrossRef - Finney D (1971). "Probit Analysis." Cambridge University Press. Cambridge, UK
- Chew, B. P. Antioxidant vitamins affect food animal immunity and health. J. Nutri.1995;125: 18045-18085.
- Nourian.K., Baghshani.H .,Shahsavani.D., (The Effect of Vitamin C on Lead-induced Plasma Biochemical Alterations in Fish, Cyprinus carpio” .Iran. J. of Toxicol 2019 13(2): 25-29
CrossRef - Hedayati A, A Safahieh, A Savari and GJ Marammazi. Assessment of aminotransferase enzymes in yellowfin sea bream (Acanathopargus latus) under experimental condition as biomarker of mercury pollution. World J Fish Marine Sci. 2010;186-192.
- Reddy, S. J. Immunostimulatory Effect of Supplementary Diet Vitamin C on Growth, Haematology, Survival and Immunity of Fish, Catla Catla. IOSR Journal Of Pharmacy. 2018; (8)12:46-54.
- Osawa T, Kato Y. Protective role of antioxidative food factors in oxidative stress caused by hyperglycemia. Ann NY Acad Sci. 2005; 1043:440-451.
CrossRef - Jenkins, F., Smith, J., Rajanna, B., Shameem, U., Umadevi, K., Sandhya, V., Madhavi, R. Effect of sub lethal concentration of endosulfan on hematological and serum biochemical parameters in the carp, Cyprinus carpio. Bull. Environ. Contam. Toxicol. 2003; 70:993–997.
CrossRef - Ibrahim, R.. E, Ahmed S. A.A, Amer, S. A, Al-Gabri, Naif A, Ahmed. A. I, AbdelWarith. A. A, Younis, E.M.I, Metwally, A.E. Influence of vitamin C feed supplementation on the growth, antioxidant activity, immune status, tissue histomorphology, and disease resistance in Nile tilapia, Oreochromis niloticus. Aquaculture Reports. 2020; 100545.
CrossRef - Sharaf. M. A, Farrag. H.R.A, Fahmy.M.H. Protective Effects of Vitamin C on Hematological and Biological Parameters of Intoxicated Male Albino Rats With Lead and Cadmium.Middle East J. Appl. Sci .2017;7(1): 57-67.
- T S Gill , A. Epple. Stress-related changes in the Hematological profile of the American eel Anguilla rostrata .1993; 25(2):227-35.
CrossRef






