Plant Growth-Promoting Bacteria (PGPB): A Potent Source of Heavy Metal Stress Management in Plants
1
Microbiology Laboratory, Department of Life Sciences,
Dibrugarh University,
Dibrugarh,
Assam
India
2
Department of Life Sciences,
Dibrugarh University,
Dibrugarh,
Assam
India
Corresponding author Email: ratulnath@dibru.ac.in
DOI: http://dx.doi.org/10.12944/CWE.18.3.30
Copy the following to cite this article:
Gogoi A, Borah N, Nath R. Plant Growth-Promoting Bacteria (PGPB): A Potent Source of Heavy Metal Stress Management in Plants. Curr World Environ 2023;18(3). DOI:http://dx.doi.org/10.12944/CWE.18.3.30
Copy the following to cite this URL:
Gogoi A, Borah N, Nath R. Plant Growth-Promoting Bacteria (PGPB): A Potent Source of Heavy Metal Stress Management in Plants. Curr World Environ 2023;18(3).
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2023-06-22 |
|---|---|
| Accepted: | 2023-10-29 |
| Reviewed by: | 
 Jogendra Singh
Jogendra Singh
|
| Second Review by: |

 Vidya. P
Vidya. P
|
| Final Approval by: | Dr. Vindhya Prasad Tewari |
Introduction
Heavy metals (HMs) are naturally occurring toxic elements widely distributed in the environment. Due to some anthropogenic activities, the concentration of the HMs on the earth’s surface is increasing gradually, sometimes crossing the normal limits, causing remarkable harm to the environment 1. Heavy metals, after entering the soil, their persistence become a long-term threat to soil microbiota and vegetation, resulting in ecosystem malfunction. HMs in the soil may be present due to natural processes or anthropogenic activities. In natural processes, HMs in soil are derived from soil parent materials such as metal-enriched rocks, serpentine, and black shale, etc. The anthropogenic sources of heavy metals tend to be more mobile than the natural lithogenic sources 2. Mining, smelting, sewage & sludge supplementation (biosolids), application of agrochemicals and lead-based paints, leaded petrochemicals, etc. are the major anthropogenic sources of HM in the soil 3.
Pb, Ni, Hg, Cr, Cu, As, Cd, Zn, etc. are the most common heavy metals responsible for contamination of agricultural soil 3. In spite of their essence being in trace amounts, some HMs are poisonous to the living system at their higher concentrations. Others may be toxic in lesser amounts also. The level of HMs in the soil beyond certain limits exhibits toxic effects on plants and human health 4. In addition to contamination of food chains, absorption of HMs by plants results in chlorosis, inhibition of growth and photosynthesis, low biomass accumulation, altered water balance, etc., and at greater quantities, it leads to the death of the plant 5. Heavy metals, when they reach the water bodies, their removal by natural processes becomes very difficult and time consuming. In such cases, it may stimulate the ROS (Reactive Oxygen Species) formation causing remarkable damage to aquatic organisms 6.
Microorganisms in the soil play a pivotal role in preserving soil fertility and plant productivity. PGPB holds a key position in this environment by promoting plant growth through fixing nitrogen, controlling detrimental microorganisms, enhancing soil nutrients, and helping plants cope with various stresses in both natural ecosystems and agriculture. The rhizosphere, where plants and microorganisms interact, is a dynamic ecosystem with a diverse array of impacts on both the partners. PGPR are a group of PGPB that colonize specially the rhizosphere and augment plant growth and development via multiple pathways, including mitigation of HM stress. The ability of various PGPB species to support heavy metal cleaning, and enhance crop performance under abiotic stress has been discovered. Numerous PGPB have shown the potential to bioremediate heavy metals from contaminated soils, including Mesorhizobium sp., Burkholderia phytofirmans, Variovorax paradoxus, Bacillus pumilus, Azotobacter spp., P. libanensis, and P. reactants, 7. Due to their biological characteristics, PGPB may develop a tolerance to HMs or use direct detoxification, leading to resistance. The presence of Bacillus thuringiensis, for example, boosted the efficiency of the Alnus firma in removing metals like Zn, Cd, As, Cu, Pb, and Ni, or reduced their harmful effects by accumulating these metals in the seedlings of this plant 8. PGPB have an excellent potential to promote plant growth via different pathways, including the synthesis of plant growth regulators (IAA, GA3), production of ACC Deaminase, solubilisation of minerals (N, P) etc. For instance Cellulosimicrobium sp., with various plant growth promoting traits enhanced growth of Alfalfa under metal stress conditions 9. The elevated concentration of HM in the ecosystem significantly affects the microbial communities 10. Many bacteria die due to exposure to these contaminants, despite the fact that some microbes, like PGPB, have evolved numerous defense mechanisms against the toxicity of HMs. Thus, PGPB, when used as biofertilizers, helps enhance the growth of plants grown in HM-contaminated soil 11. The present review aims to study the responses of plants to HM stress, mechanisms adopted by microbes to overcome HM stress, and management of HM stress by using PGPB for sustainable agriculture.
Materials and methods
This paper uses academic databases, libraries, and online resources to gather relevant research articles, reviews, and other scholarly materials related to microbes' heavy metal stress management mechanisms and their role in managing HM stress in plants, mostly from 2010 onwards. Critical analysis of the responses of PGPB and plants to HM, mechanisms responsible for managing HM stress in plants and microbes, and application of PGPB in managing heavy metal stress are the key points selected for this article.
Responses of Plants to Heavy Metal Stress
Plants have different defense mechanisms that are activated during stress conditions; they also maintain the critical metal homeostasis required by plants 12. The responses of plants to the toxicity of different HMs vary from species to species. HMs such as Pb, As, Hg, Cd, Cr, etc encountered in contaminated soil are toxic in both chemically combined and elemental forms 13. The first line of defense of plants against toxicity is to minimize metal uptake when toxicity is encountered. This is performed with the aid of cellular and root exudates, that can change the pH of the rhizosphere 12. As a second line of defense, plants use various molecular and physiological systems, including separation, chelating metal production, accumulation, exclusion, and manufacture of osmoprotectants, etc. 14. HMs are chelated and sequestered by compounds such as phytochelatins, metallothioneins, and antioxidants such as superoxide dismutase (SOD) and peroxidase in the cytosol 15.
To survive under stress conditions, complicated signal transduction processes are activated within the plant cell. These signaling pathways help to induce the transcription of various metal stress-responsive genes. For instance, signaling pathways of hormone and ROS, MAPK (Mitogen-activated protein kinase) cascade, and Ca–Calmodulin pathway, etc. are activated in response to different metal stresses 12. In the Ca-Calmodulin pathway, Ca acts as a second messenger, eliciting responses to diverse biotic and abiotic stress signals. These signals initiate downstream events that lead to a change in gene expression like ABA (Abscisic acid)-responsive genes, MIR genes, metal transporters, etc., and the adaptation of plants to stress tolerance. In response to HM stress, NO (Nitric Oxide) is known to be associated with raising the level of Ca2+, which in turn regulates the elevation/ control of NO concentration, along with elicitation of specific physiological responses to a given signal, thus having a combined function in HM or abiotic stress regulation 16. Nitric oxide is a very effective and widely used signaling molecule responsible for reducing oxidative stress as it participates in the breakdown of oxygen radicals to hydrogen peroxide and oxygen, and it might also serve as a signal that stimulates the activities of ROS-scavenging enzyme under abiotic stress. Nitric oxide can detoxify the free radicals in the cell as it is itself an antioxidant and also promotes the synthesis of antioxidant enzymes 17. ROS not only causes damage to DNA and the cell membrane but also serve as a vital signaling chemical that regulates plant growth and plant protection against HM. It has been reported that ROS production that is induced by heavy metals is known to activate MAPK signalling, which works downstream processing of ROS 18.
Proline, an amino acid accumulated by a plant in response to HM stress acts as an osmoprotectant, ROS quencher, and HM chelator. Proline increases the tolerance of a plant to HM stress by various mechanisms. When plants are exposed to heavy metals, the activity of proline increases antioxidant enzyme activities, reconstruction of Chlorophyll, as well as regulation of intracellular pH, etc 19. The formation of phytochelatin that can chelate HM to decrease its toxicity is induced by Proline 20. According to Xu et al. (2009) pre treatment of proline protected the plasma membrane of the callus subjected to Cd stress by reducing the level of ROS, thereby improving the Cd tolerance in Solanum nigrum. It has been shown that, inhibition of enzyme activity (glucose-6-phosphate dehydrogenase and nitrate reductase) caused by Cd and Zn was protected by exogenous application of proline 20.
Various studies have shown that phytohormones play a vital role during stress. Exposure of plants to HM intensifies complicated signal transduction networks and synthesizes stress-related phytohormones 22. Some of the plant hormones related to heavy metal contamination are Gibberellic acid (GA), Auxin (IAA), Abscisic acid (ABA), ethylene, etc. Abscisic acid is an essential phytohormone that has been linked with tolerance to adverse environmental conditions having a role in abiotic stresses. Endogenous ABA concentration in plant tissue is known to rise in response to HM exposure, which, by activating specific signaling pathways, modulates gene expression levels in plants 15. Exogenous ABA enhances tolerance to excess Zn and increases the expression of a gene to HM detoxification. A previous experiment has shown an increase in ABA levels in the roots of Phragmites and Typha when they are treated with Cd and also noted the involvement of ABA in the activation of O-acetyl serine, which is responsible for cysteine biosynthesis 23.
IAA is reported as an important mediator for plant growth and development in both regular stressfull conditions. Auxin homeostasis within the plant may be disturbed by HM, for instance, Cd stress leads to change in auxin homeostasis in Arabidopsis seedlings 23. Exogenous application of IAA alleviates HM stress while maintaining endogenous IAA homeostasis 25. To alleviate the effect of HM toxicity in plants, auxin has been observed to cross-talk with the ROS detoxification system. As (Arsenic) toxicity in Arabidopsis, elevates the H2O2 content which is responsible for promoting the transportation of auxin through AUX1. Additionally, it causes a decrease in the transcript levels of catalase-3 (CAT3). Thus the cross-talk between auxin and ROS could potentially be crucial in the mechanisms that enable tolerance to HM stress 22,26.
Ethylene is a gaseous phytohormone that is involved in numerous biological processes such as floral senescence, abscission of leaves, ripening of fruit, etc. When the plant faces HM stresses, the rate of ethylene synthesis increases, which is associated with a decline in plant growth. In Arabidopsis, Cd induces the activation of ethylene biosynthesis genes ACS2 [1-aminocyclopropane-1-carboxylic acid (ACC) synthase] and ACS6, which leads to an increase in ethylene production via Yang's cycle. On the contrary, the acs2-1acs6-1 double knockout mutant failed to display higher ethylene production under Cd stress 15,27.
GA (Gibberellic acid) is responsible for seed germination, stem elongation, fruit development, etc. They promote plant tolerance levels to HM stress and enhance antioxidant effectiveness, thereby minimizing the toxicity of metals like Cd, Ni, Cr, and Fe, etc. Degradation of DELLA (Aspartic acid, Glutamic acid, Leucine and Alanine) a negative regulator of GA signaling protein is induced by GA. It has been noted that under Cd and Pb stress, exogenous application of GA in Chlorella vulgaris increased protein content and cell number 14.
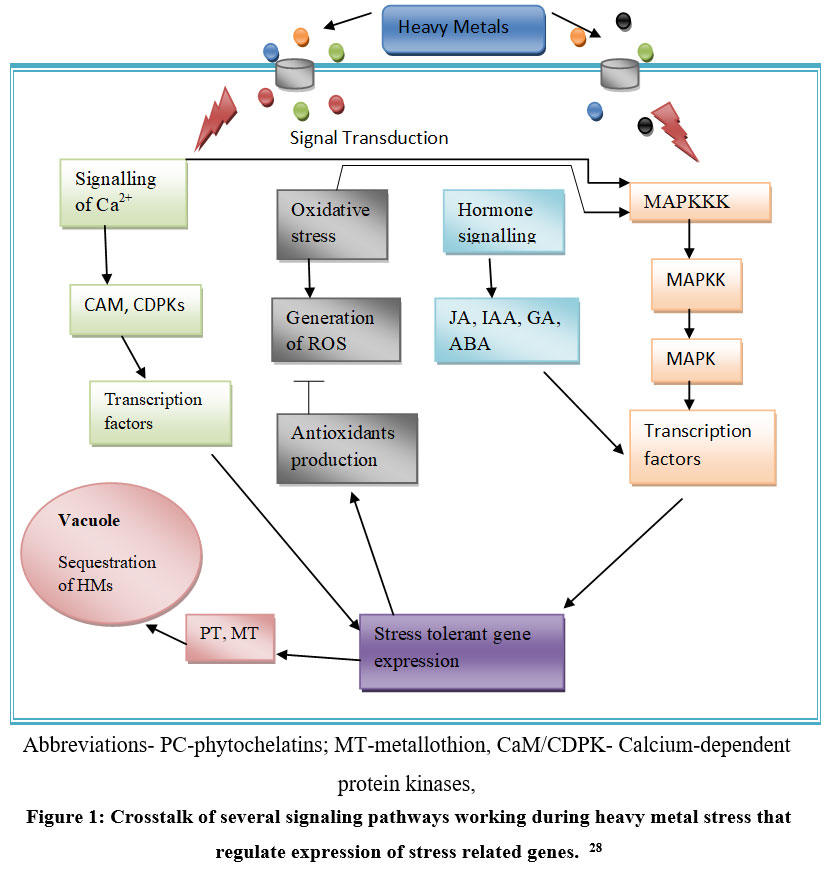 | Figure 1: Crosstalk of several signaling pathways working during heavy metal stress that regulate expression of stress related genes.28
|
Heavy Metal Resistant Soil Microorganisms
The huge amounts of mine waste reduce the biological activity of soil microorganisms due to the discharge of HMs from the minerals by high amount of sulfuric acid. It has been observed that HM contamination affects the microbial communities both for shorter and longer durations. However, different microbial communities have varying levels of resistance to soil heavy metal toxicity 29. Microorganisms have a vital function in the remediation of environments contaminated with HMs through the biogeochemical cycling of metals. Microbes can alter the mobility and bioavailability of HMs by releasing chelating substances (siderophores), dissolving metal phosphate, altering redox potentials, and acidifying soil 29,30. It was established that the population and occurrence of HM-resistant bacteria increased with increasing concentrations of HM 31. They have the ability to reduce the toxicity level of HM. Additionally, by bringing certain HMs down to a lower redox state, microorganisms can keep them out of polluted soils. These microbes are renowned as dissimilatory metal-reducing bacteria. In anaerobic respiration, they utilize metals as terminal electron acceptors in anaerobic respiration 11. EPS (extracellular polymeric substances) are fundamental constituents of biofilms that provide support and protect the microbial communities from harsh environmental conditions. These substances potentially increase microbial resistance to HM concentrations. These are composed mainly of polysaccharides but may also include proteins, extracellular DNA, lipids, and humic substances. EPS have the capability to remove HM and emulsify hydrophobic compounds in the remediation process 31.
Heavy metal resistance mechanism of bacteria
Tolerance of bacteria to HM stress depend upon several factors such as localization of metal resistance genes, types of metal ion transport into the cell, etc. There are five main mechanisms of HM-resistant bacteria. These are-
Extracellular barrier
A bacterial cell’s capsule, it’s cell wall, and plasma membrane act as an extracellular shield to inhibit the entry of metal or metal ions into the cell. Metal ions can be absorbed by some bacteria via the ionizable cell walls or capsule groups such as phosphate, amino, carboxyl, and hydroxyl groups). Several authors have found that non-viable cells of some bacterial species, like Bacillus sp., Pseudomonas putida, and Brevibacterium sp., possess a significant amount of passive biosorption of HM ions 32. The plasma membrane’s altered permeability may restrict metal ions from entering the cell. Silver ion accumulation inside the cell was found to be low in Esch?richia coli mutants that lack porins, which are membrane proteins that operate as transport channels for hydrophilic substances 33. Gram-positive bacteria have a thick murein coating that can keep a hazardous component out of the cell. Due to the presence of mycolic acid, some specific gram-positive genera, including Dietzia, Corynebacterium, Rhodococcus, Nocardia, Tsukamurell, and Skermania, are incredibly resistant to poisonous substances 34.
Active transport of metal ions
Efflux pump system or active transport corresponds to protein-rich transport and is represented as the largest group of HM resistance systems of bacteria. They exploit this mechanism to decrease the accumulation potential and concentration of cellular detoxification. Some metal ions are transported inside the cell via a system liable for the absorption of vital components. Previous studies have shown that Cd, Co, Zn, etc. enter the cell via the Mg transport system of Alcaligenes eutrophus 33.
Efflux systems containing proteins are afflicted into five major families, comprising the major facilitator superfamily, the small multidrug resistance family included in the larger drug/metabolite transporter superfamily, the RND family (Resistance, Nodulation, division), the multidrug and toxic compound extrusion family, and the ATP-binding cassette superfamily 36. These efflux pumps are energy-dependent as they transport substrates against the concentration gradient. They draw energy from ATP hydrolysis or from chemical gradients. ABC transporters play a significant role in nutrient uptake and in the expulsion of harmful substances from the cell, and are considered as an important virulence factor, they also secrete peptides, lipids, hydrophobic drugs, etc 37. P-type ATPase and CDF (cation diffusion facilitators) proteins are involved in bacterial immunity 34,37. P-type ATPase mainly transfers metal ions with high affinity, while CDF-proteins specifically interact with divalent metal ions. Metal-efflux is a mechanism by which membrane-bound CDF proteins contribute to bacterial metal tolerance. The RND protein family has tripartite organizations that transport HMs, proteins, and other substances from the periplasm across the plasma membrane 37,38
Extracellular sequestration
Extracellular sequestration describes the complexation of metal ions into insoluble compounds or the accumulation of metal ions by cellular components in the periplasm or the cell’s outer membrane 32. This process involves the secretion of chelating agents like phosphate, siderophores, sulfide, oxalate etc 38. Copper-resistant strains Pseudomonas syringae synthesize membrane proteins such as CopA, CopB, and CopC that can bind to copper ions as an outcome of metal accumulation. Studies have shown that the accumulation of copper by a resistant strain was in a complex form, whereas the accumulation of copper done by a sensitive strain in a free ionic form is highly hazardous to the cell 33.
Intracellular sequestration
Intracellular sequestration refers to the complexation of metal ions by various compounds in the cytoplasm of a cell. As a result of association with ligands on the surface followed by sluggish transport, the metal concentration of the cell could rise. Through the influx mechanism, HM detoxification by bacterial cells is developed, and metallothioneins sequester it intracellularly 37. The cyanobacterium Synechococcus sp. PCC 7942 has provided evidence that prokaryotic cells can synthesize metallothionein and is encoded by the genes smtA and smtB, which are triggered by cadmium and zinc ions 40. With the aid of cysteine-rich proteins, the cadmium-tolerant strain Pseudomonus putida is able to sequester Cu, Cd, and Zn ions inside its cells. In Rhizobium leguminosarum cells, glutathione was found to intracellularly sequester Cd ions 41.
Reduction of metal ions
By changing the oxidation states of metal ions, microbial organisms can lessen their noxiousness. Some bacteria produce energy by using metallic elements and metalloids as electron donors or acceptors. During bacterial anaerobic respiration, oxidized metals might behave as terminal acceptors of electrons 32,33. Enzymatic metal ion reduction could lead to the creation of less hazardous forms of chromium and mercury. For instance, some Bacillus sp can reduce the toxicity level of Pb by transforming it from Pb(NO3) to a less toxic PbS form 37. Reduction or biotransformation of toxic Cd to Cds by Pseudomonas aeruginosa provided an eco-friendly circumvention for toxicity removal, as reported by Mahle et al., (2020).
HM Resistance Genes in Bacteria
The mechanisms applied by microbes in response to HM stress are encoded by genes present in chromosomes and on plasmids. Several Metal Resistance Genes (MRG) are reported in bacterial communities from wide habitats 44. Abou-Shanab et al., (2007) isolated forty-five bacteria from Ni-rich soil and analyzed several metal-resistant genes present in those bacteria, specifically five cultures such as Rhizobium mongolense, Arthrobacter rhombi, Clavibacter xyli, Variovorax paradoxus, and Microbacterium arabinogalactanolyticum were tolerant to nine different metals. They found that ncc, czc, chr, and mer genes are responsible for resistance to Cr, Ni, Zn, and Hg by using different molecular techniques such as PCR, RFLP, and DNA-DNA hybridization. According to Abdelatey et al., (2011) both gram-positive and gram-negative bacteria, including Staphylococcus aureus, Bacillus subtilis, Bacillus cereus, Pseudomonas sp., and Bordetella sp., showed metal tolerance against Cd2+ and Co2+. The MRGs such as chr, czc, mer, and ncc were shown to be present in these bacteria by using the semi-quantitative reverse transcription-PCR. Previously, Pseudomonas putida was isolated from sewage sludge samples, which were found to be resistant to Cd. The MRG involved in tolerance to Cd came from three gene clusters such as czcCBA1, cadA2R, and colRS 47. Some more Gram-negative bacteria, like Pseudomonas aeruginosa and Cupriavidus metallidurans have the ability to tolerate Cd, and the czcABC gene is responsible for (cobalt/zinc/cadmium) resistance. The czc gene cluster identified in Alcaligenes eutrophus was plasmid-encoded, whereas the homologous gene cluster (czc) called czr identified in Pseudomonas aeruginosa was chromosomal coded and resistant to Cd, Zn, and Co 48.
Application of HM-Resistant PGPB in Plant Stress Management
The association between plants and microorganisms may involve several mechanisms that are important for both plants and microbial communities, this interaction may be harmful, beneficial, or neutral. Among rhizosphere microorganisms, PGPB may directly enhance plant growth by either regulating phytohormone levels or facilitating resource acquisition, or indirectly by acting as a biocontrol agent 50.
Bacteria are the most crucial microorganisms for the treatment of soil contaminated with HMs. Several studies have demonstrated the role of PGPB in the elevation of crop yield either directly or indirectly due to their stimulatory impacts on soil nutrients, boosted nutrient uptake, overall physiological processes, and coping with stressfull situations by plants, and plants’ resistance against pathogens 7,50–52. The mechanism by which PGPB influences plant development and growth differs among species or strains; that includes restoration of soils, nitrogen and phosphate solubilization, and generation of plant hormones and siderophores, among others 7. By generating 1-aminocyclopropane-1-carboxylate deaminase (ACCD), which dissolves insoluble mineral nutrients like potassium, phosphorus, nitrogen, etc, PGPB can lower the metal toxicity, change the bioavailability of metal in soils, and improve both abiotic and biotic stress resilience 53. Rhizobium, Pseudomonas, Azospirillum, Alcaligenes, Bacillus, Arthrobacter, Agrobacterium, Azotobacter, Burkholderia, Klebsiella, and Enterobacter species are among a group of PGPB that are resistant to metals and have the potential to promote plant growth in metal-contaminated soil 53. It has been reported that Pseudomonas fluorescens can enhance the growth of Sedum alfredii when exposed to stressors Zn and Cd by generating IAA 54.
PGPB can also increase plant growth and development under HM-stress situations by fixing nitrogen, dissoluting phosphorus and potassium. For instance, Klebsiella variicola, one of the PGPB can increase the bioavailability of phosphate in the rhizosphere by transforming insoluble phosphate to soluble form with the aid of enzymes C–P lyases and phosphonates 55.
The stress hormone ethylene acts at low concentrations and regulates plant growth and development. Many recent investigations aim to minimize the ethylene level in plants through the activity of the bacterial enzyme ACCD which controls the ethylene generation by converting ACC into ?-keto butyric acid and ammonia 56.
Metal bioavailability in soil can be reduced by the combination of the metal with extracellular substances, thereby reducing metal absorption by plants through the root system. This mechanism is done by some PGPB through precipitation, alkalization, and complexation processes. To form insoluble precipitates, PGPB secretes some inorganic acids that can react with dissolved metals like Cu, Fe, Zn, Pb, etc 57.
Siderophores (chelator agents) are produced by bacteria to overcome nutritional Fe limitations as they have a high affinity for chelating Fe3+. Thus, they aid in the enhancement of plants growth and also protect against phytopathogen. Depending on their chemical nature, siderophores are divided into different groups that include, catecholates, hydroxamates, phenolates, carboxylates, and mixed types. Pseudobactin and pyoverdine, a mixed type of siderophore are synthesized by various Pseudomonas sp. 58. A hydroxamate type of siderophore, mainly pyoverdine, is produced by Pseudomonas aeruginosa 61. da Costa et al., (2014) observed that the bacterial genera such as Grimontella and Burkholderia, had strains that produced a lot of siderophores, while other genera such as Stenotrophomonas, Herbaspirillum and Citrobacter had strains that produced a lot less siderophores 56. According to de Souza et al., (2013) isolates of the genera Enterobacter and Burkholderia produced the most siderophores.
Table 1: Use of PGPB in heavy metal detoxification and its role in alleviation of plant HM stress
Heavy metal | PGPB strain | Host plant | Main PGP trait | Effects | Reference |
Cu, Cd, Pb and Zn | Alcaligenes faecalis MG257493.1, Bacillus cereus MG257494.1 and Alcaligenes faecalis MG966440.1 | Sorghum bicolor, L. | Siderophores, Chelating agents, EPS | Increased plant height, photosynthetic pigments and enhanced plant growth. | 62 |
Cr | Myroides odoratimimus TCR22, B. cereus TCR17, Providencia rettgeri TCR21, | Sorghum bicolour | IAA, siderophores. | Stimulated plant growth, increased pigment contents, protein, antioxidant (Superoxide Dismutase, Catalase). | 63 |
Zn | Serratia sp. ZTB | Zea mays | IAA, siderophores, ACCD, and solubilisation of phosphate(P) and potassium(K) | Enhanced plant growth, improved antioxidant enzyme activities. Under Zn stress, accumulation of Zn was reduced in maize plantlet. |
64 |
Cd | B. contaminans | Glycine max | P-solubilization, ACCD, siderophores, and IAA | Increased nitrogen content and plant tolerance to Cd, promoted plant dry biomass. | 65 |
Cd | P. fluorescens | Sedum alfredii | IAA | promoted a lateral root formation of its host plant, efficiency of higher Cd phytoremediation. | 66 |
Zn, Al and Pb | Halobacillussp. SB2, Bacillus sp. SB1 | Arachis hypogaea | N2-fixation, P solubilisation | It had a positive impact on different plant physiological processes. | 67 |
Ni | Psychrobacter sp., Bacillus cereus SRA10, Bacillus weihenstephanensis SRP12 | B. juncea, B. Oxyrrhina | Siderophore, ACCD, IAA, P- solubilisation | Directly improves phytoextraction efficiency by increasing metal accumulation in plant tissues. | 68 |
Cu, Cd, Pb | Ochrobactrum cytisi, Bradyrhizobium sp. 750, | Lupinus luteus | N 2 fixation | Boost plant yield and N-content, and lower plant metal accumulation. | 69 |
Mn | B. thuringiensis, B. cereus | Broussonetia papyrifera | Siderophores IAA, and P solubilization, | Enhanced biomass, increased total length of root, surface area of the plant and also improved soil environment. | 70 |
Co, Cd, Cu, Cr, Ni, Zn | B. vietnamensis AB403, Kocuria flava AB402 | O. sativa |
EPS, IAA, siderophores | increased rice seedlings growth in As-amended hyper saline soil. | 71 |
Cd, Zn | Rhodobacter sphaeroides | T. aestivum | IAA | Decreased the metal accumulation in plants. | 72 |
Cu | P. thivervalensis, B. Cepacia, Microbacterium oxydans, | Brassica napus | Siderophores, P solubilisation, IAA, ACCD | Increased the antioxidant contents such as Ascorbic acid, Glutathione, enhanced plant biomass. | 73 |
Cd | Serratia sp. | Zea mays | IAA, P-solubilization | Enhanced plant growth ,increased biomass accumulation, decreased ROS production. | 74 |
Cu, Cr, Cd | P. aeruginosa CPSB1 | T. aestiv um | IAA, HCN, siderophore, ACCD, P solubilisation | Enhanced production of wheat, showed metal tolerance capability. | 75 |
Cd, Cu, Zn, and Pb | Streptomyces pactum Act 12 | Triticum aestivum |
IAA, ACCD, siderophores
| Promoted plant growth, raised plant biomass and decreased antioxidant activities. | 76 |
Cd | Pseudomonas fluorescence PGPR-7 and Trichoderma sp. T-4 | Cicer aeietinum |
Siderophore | Enhanced plant growth, increased seed germination, content of Chlorophyll and carotenoid. | 77 |
Discussion and Conclusion
The review has demonstrated that PGPB play a pivotal role in enhancing HM tolerance in plants. Various mechanisms employed by PGPB, such as enhancing nutrient uptake, metal chelation, and reducing HM uptake and translocation, contribute to reducing the toxic effects of HM on plants. This not only improves the overall health and growth of plants but also helps in maintaining crop productivity under HM-contaminated conditions. The utilization of PGPB as biofertilizers and bioremediation agents offers a promising and environmentally friendly alternative to traditional approaches for managing HM stress in plants. Continued research development in this field hold the potential to transform agriculture and contribute to sustainable environmental conservation.
Due to fast industrialization, sophisticated agricultural practices, and expanding anthropogenic activities, the toxicity of HM in soil has now emerged as one of the most important issues in the globe’s history. A lot of work or experiments have been done to decrease, eliminate, and deteriorate the HMs from the soil. Heavy metal accumulation in plants affects various biological functions like hormonal imbalance within plants. The exogenous application of phytohormone boosts the yields and production of crops exposed to HM. However, some microorganisms present in soil can minimize the toxicity level of HM. Among them, PGPB may directly enhance the growth and development of plant by various processes. The use of these PGPBs has great potential in the remediation of HM-contaminated sites. Thus further studies are required in the future to find more beneficial bacteria that can diminish stress.
Acknowledgement
We sincerely acknowledge the Department of Life Sciences, Dibrugarh University for providing infrastructure facilities. We are also thankful to all the scholars of microbiology laboratory for their help and support.
Conflict of Interest
There is no conflict of interest between authors.
Funding Sources
No fund was received for the present work.
Reference
- Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ. Heavy Metal Toxicity and the Environment. In: Luch A, ed. Molecular, Clinical and Environmental Toxicology: Volume 3: Environmental Toxicology. Experientia Supplementum. Springer; 2012:133-164. doi:10.1007/978-3-7643-8340-4_6.
CrossRef - Wuana RA, Okieimen FE. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation. ISRN Ecol. 2011;2011:1-20. doi:10.5402/2011/402647
CrossRef - Smiljani? S, Tomi? N, Perusic M, Vasiljevi? L, Pelemis S. THE MAIN SOURCES OF HEAVY METALS IN THE SOIL AND PATHWAYS INTAKE. In: ; 2019. doi:10.7251/EEMEN1901453S
CrossRef - Li C, Zhou K, Qin W, et al. A Review on Heavy Metals Contamination in Soil: Effects, Sources, and Remediation Techniques. Soil Sediment Contam Int J. 2019;28(4):380-394. doi:10.1080/15320383.2019.1592108
CrossRef - Singh S, Parihar P, Singh R, Singh VP, Prasad SM. Heavy Metal Tolerance in Plants: Role of Transcriptomics, Proteomics, Metabolomics, and Ionomics. Front Plant Sci. 2016;6:1143. doi:10.3389/fpls.2015.01143
CrossRef - Singh DrJ, Kalamdhad A. Effects of Heavy Metals on Soil, Plants, Human Health and Aquatic Life. Int J Res Chem Environ. 2011;1:15-21.
- Majeed A, Muhammad Z, Ahmad H. Plant growth promoting bacteria: role in soil improvement, abiotic and biotic stress management of crops. Plant Cell Rep. 2018;37(12):1599-1609. doi:10.1007/s00299-018-2341-2
CrossRef - Poria V, D?biec-Andrzejewska K, Fiodor A, et al. Plant Growth-Promoting Bacteria (PGPB) integrated phytotechnology: A sustainable approach for remediation of marginal lands. Front Plant Sci. 2022;13:999866. doi:10.3389/fpls.2022.999866
CrossRef - Tirry N, Tahri Joutey N, Sayel H, et al. Screening of plant growth promoting traits in heavy metals resistant bacteria: Prospects in phytoremediation. J Genet Eng Biotechnol. 2018;16(2):613-619. doi:10.1016/j.jgeb.2018.06.004
CrossRef - Eltahawy AMAE, Awad ESAM, Ibrahim AH, Merwad ARMA, Desoky ESM. Integrative application of heavy metal–resistant bacteria, moringa extracts, and nano-silicon improves spinach yield and declines its contaminant contents on a heavy metal–contaminated soil. Front Plant Sci. 2022;13:1019014. doi:10.3389/fpls.2022.1019014
CrossRef - Ahemad M. Remediation of metalliferous soils through the heavy metal resistant plant growth promoting bacteria: Paradigms and prospects. Arab J Chem. 2019;12(7):1365-1377. doi:10.1016/j.arabjc.2014.11.020
CrossRef - Ghori NH, Ghori T, Hayat MQ, et al. Heavy metal stress and responses in plants. Int J Environ Sci Technol. 2019;16(3):1807-1828. doi:10.1007/s13762-019-02215-8
CrossRef - Shah FUR, Ahmad N, Masood KR, Peralta-Videa JR, Ahmad F ud D. Heavy Metal Toxicity in Plants. In: Ashraf M, Ozturk M, Ahmad MSA, eds. Plant Adaptation and Phytoremediation. Springer Netherlands; 2010:71-97. doi:10.1007/978-90-481-9370-7_4
CrossRef - Saini S, Kaur N, Pati PK. Phytohormones: Key players in the modulation of heavy metal stress tolerance in plants. Ecotoxicol Environ Saf. 2021;223:112578. doi:10.1016/j.ecoenv.2021.112578
CrossRef - Bücker-Neto L, Ana Luiza Sobral Paiva, Machado RD, Arenhart RA, Margis-Pinheiro M. Interactions between plant hormones and heavy metals responses. Genet Mol Biol. 2017;40(1 suppl 1):373-386. doi:10.1590/1678-4685-GMB-2016-0087
CrossRef - Khan MN, Mohammad F, Mobin M, Saqib MA. Tolerance of Plants to Abiotic Stress: A Role of Nitric Oxide and Calcium. In: Khan MN, Mobin M, Mohammad F, Corpas FJ, eds. Nitric Oxide in Plants: Metabolism and Role in Stress Physiology. Springer International Publishing; 2014:225-242. doi:10.1007/978-3-319-06710-0_14
CrossRef - Mh S, Mh AW, Mo B. Role of nitric oxide in tolerance of plants to abiotic stress. Protoplasma. 2011;248(3). doi:10.1007/s00709-010-0206-9
CrossRef - Pitzschke A, Djamei A, Bitton F, Hirt H. A Major Role of the MEKK1–MKK1/2–MPK4 Pathway in ROS Signalling. Mol Plant. 2009;2(1):120-137. doi:10.1093/mp/ssn079
CrossRef - Emamverdian A, Ding Y, Mokhberdoran F, Xie Y. Heavy Metal Stress and Some Mechanisms of Plant Defense Response. Sci World J. 2015;2015:1-18. doi:10.1155/2015/756120
CrossRef - Hayat S, Hayat Q, Alyemeni MN, Wani AS, Pichtel J, Ahmad A. Role of proline under changing environments. Plant Signal Behav. 2012;7(11):1456-1466. doi:10.4161/psb.21949
CrossRef - Xu J, Yin H, Li X. Protective effects of proline against cadmium toxicity in micropropagated hyperaccumulator, Solanum nigrum L. Plant Cell Rep. 2009;28(2):325-333. doi:10.1007/s00299-008-0643-5
CrossRef - Nguyen TQ, Sesin V, Kisiala A, Emery RJN. Phytohormonal Roles in Plant Responses to Heavy Metal Stress: Implications for Using Macrophytes in Phytoremediation of Aquatic Ecosystems. Environ Toxicol Chem. 2021;40(1):7-22. doi:10.1002/etc.4909
CrossRef - Pál M, Janda T, Szalai G. Interactions between plant hormones and thiol-related heavy metal chelators. Plant Growth Regul. 2018;85(2):173-185. doi:10.1007/s10725-018-0391-7
CrossRef - Hu YF, Zhou G, Na XF, et al. Cadmium interferes with maintenance of auxin homeostasis in Arabidopsis seedlings. J Plant Physiol. 2013;170(11):965-975. doi:10.1016/j.jplph.2013.02.008
CrossRef - Noor I, Sohail H, Sun J, et al. Heavy metal and metalloid toxicity in horticultural plants: Tolerance mechanism and remediation strategies. Chemosphere. 2022;303:135196. doi:10.1016/j.chemosphere.2022.135196
CrossRef - Krishnamurthy A, Rathinasabapathi B. Auxin and its transport play a role in plant tolerance to arsenite-induced oxidative stress in Arabidopsis thaliana. Plant Cell Environ. 2013;36(10):1838-1849. doi:10.1111/pce.12093
CrossRef - Emamverdian A, Ding Y, Mokhberdoran F, Ahmad Z. Mechanisms of Selected Plant Hormones under Heavy Metal Stress. Pol J Environ Stud. 2020;30:497-507. doi:10.15244/pjoes/122809
CrossRef - Jalmi SK, Bhagat PK, Verma D, et al. Traversing the Links between Heavy Metal Stress and Plant Signaling. Front Plant Sci. 2018;9:12. doi:10.3389/fpls.2018.00012
CrossRef - Hemmat-Jou MH, Safari-Sinegani AA, Mirzaie-Asl A, Tahmourespour A. Analysis of microbial communities in heavy metals-contaminated soils using the metagenomic approach. Ecotoxicology. 2018;27(9):1281-1291. doi:10.1007/s10646-018-1981-x
CrossRef - Xie Y, Bu H, Feng Q, et al. Identification of Cd-resistant microorganisms from heavy metal-contaminated soil and its potential in promoting the growth and Cd accumulation of bermudagrass. Environ Res. 2021;200:111730. doi:10.1016/j.envres.2021.111730
CrossRef - Domingues VS, de Souza Monteiro A, Júlio ADL, Queiroz ALL, dos Santos VL. Diversity of Metal-Resistant and Tensoactive-Producing Culturable Heterotrophic Bacteria Isolated from a Copper Mine in Brazilian Amazonia. Sci Rep. 2020;10(1):6171. doi:10.1038/s41598-020-62780-8
CrossRef - Igiri BE, Okoduwa SIR, Idoko GO, Akabuogu EP, Adeyi AO, Ejiogu IK. Toxicity and Bioremediation of Heavy Metals Contaminated Ecosystem from Tannery Wastewater: A Review. J Toxicol. 2018;2018:2568038. doi:10.1155/2018/2568038
CrossRef - Ianieva O. [Mechanisms of bacteria resistance to heavy metals]. Mikrobiolohichny? Zhurnal Kiev Ukr 1993. 2009;71:54-65.
- Murínová S, Dercová K. Response Mechanisms of Bacterial Degraders to Environmental Contaminants on the Level of Cell Walls and Cytoplasmic Membrane. Int J Microbiol. 2014;2014:e873081. doi:10.1155/2014/873081
CrossRef - Sharma A, Gupta VK, Pathania R. Efflux pump inhibitors for bacterial pathogens: From bench to bedside. Indian J Med Res. 2019;149(2):129-145. doi:10.4103/ijmr.IJMR_2079_17
CrossRef - Mathivanan K, Chandirika JU, Vinothkanna A, Yin H, Liu X, Meng D. Bacterial adaptive strategies to cope with metal toxicity in the contaminated environment – A review. Ecotoxicol Environ Saf. 2021;226:112863. doi:10.1016/j.ecoenv.2021.112863
CrossRef - Valencia EY, Braz VS, Guzzo C, Marques MV. Two RND proteins involved in heavy metal efflux in Caulobacter crescentus belong to separate clusters within proteobacteria. BMC Microbiol. 2013;13(1):79. doi:10.1186/1471-2180-13-79
CrossRef - Pal A, Bhattacharjee S, Saha J, Sarkar M, Mandal P. Bacterial survival strategies and responses under heavy metal stress: a comprehensive overview. Crit Rev Microbiol. 2022;48(3):327-355. doi:10.1080/1040841X.2021.1970512
CrossRef - Mathivanan K, Chandirika JU, Vinothkanna A, Yin H, Liu X, Meng D. Bacterial adaptive strategies to cope with metal toxicity in the contaminated environment – A review. Ecotoxicol Environ Saf. 2021;226:112863. doi:10.1016/j.ecoenv.2021.112863
CrossRef - Chatterjee S, Kumari S, Rath S, Priyadarshanee M, Das S. Diversity, structure and regulation of microbial metallothionein: metal resistance and possible applications in sequestration of toxic metals. Metallomics. 2020;12(11):1637-1655. doi:10.1039/d0mt00140f
CrossRef - Lima A, Corticeiro S, Figueira E. Glutathione-mediated cadmium sequestration in Rhizobium leguminosarum. Enzyme Microb Technol. 2006;39:763-769. doi:10.1016/j.enzmictec.2005.12.009
CrossRef - Mathivanan K, Chandirika JU, Vinothkanna A, Yin H, Liu X, Meng D. Bacterial adaptive strategies to cope with metal toxicity in the contaminated environment – A review. Ecotoxicol Environ Saf. 2021;226:112863. doi:10.1016/j.ecoenv.2021.112863
CrossRef - Mahle R, Kumbhakar P, Pramanik A, et al. Probing the bacterial detoxification of cadmium to form cadmium sulfide quantum dots and the underlying mechanism. Mater Adv. 2020;1(5):1168-1175. doi:10.1039/D0MA00105H
CrossRef - Tiwari A, Gomez-Alvarez V, Siponen S, et al. Bacterial Genes Encoding Resistance Against Antibiotics and Metals in Well-Maintained Drinking Water Distribution Systems in Finland. Front Microbiol. 2022;12. Accessed March 30, 2023. https://www.frontiersin.org/articles/10.3389/fmicb.2021.803094
CrossRef - Abou-Shanab RAI, van Berkum P, Angle JS. Heavy metal resistance and genotypic analysis of metal resistance genes in gram-positive and gram-negative bacteria present in Ni-rich serpentine soil and in the rhizosphere of Alyssum murale. Chemosphere. 2007;68(2):360-367. doi:10.1016/j.chemosphere.2006.12.051
CrossRef - Abdelatey L, Khalil W, Ali T, Mahrous K. Heavy metal resistance and gene expression analysis of metal resistance genes in gram-positive and gram-negative bacteria present in Egyptian soils. J Appl Sci Env Sanit. 2011;6.
- Hu N, Zhao B. Key genes involved in heavy-metal resistance in Pseudomonas putida CD2. FEMS Microbiol Lett. 2007;267(1):17-22. doi:10.1111/j.1574-6968.2006.00505.x
CrossRef - Chellaiah ER. Cadmium (heavy metals) bioremediation by Pseudomonas aeruginosa: a minireview. Appl Water Sci. 2018;8(6):154. doi:10.1007/s13201-018-0796-5
CrossRef - Glick BR. Plant Growth-Promoting Bacteria: Mechanisms and Applications. Scientifica. 2012;2012:e963401. doi:10.6064/2012/963401
CrossRef - Etesami H. Can interaction between silicon and plant growth promoting rhizobacteria benefit in alleviating abiotic and biotic stresses in crop plants? Agric Ecosyst Environ. 2018;253:98-112. doi:10.1016/j.agee.2017.11.007
CrossRef - Gouda S, Kerry RG, Das G, Paramithiotis S, Shin HS, Patra JK. Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiol Res. 2018;206:131-140. doi:10.1016/j.micres.2017.08.016
CrossRef - Pérez-de-Luque A, Tille S, Johnson I, Pascual-Pardo D, Ton J, Cameron DD. The interactive effects of arbuscular mycorrhiza and plant growth-promoting rhizobacteria synergistically enhance host plant defences against pathogens. Sci Rep. 2017;7(1):16409. doi:10.1038/s41598-017-16697-4
CrossRef - Wang Y, Narayanan M, Shi X, et al. Plant growth-promoting bacteria in metal-contaminated soil: Current perspectives on remediation mechanisms. Front Microbiol. 2022;13:966226. doi:10.3389/fmicb.2022.966226
CrossRef - Chen B, Luo S, Wu Y, et al. The Effects of the Endophytic Bacterium Pseudomonas fluorescens Sasm05 and IAA on the Plant Growth and Cadmium Uptake of Sedum alfredii Hance. Front Microbiol. 2017;8. Accessed March 17, 2023. https://www.frontiersin.org/articles/10.3389/fmicb.2017.02538
CrossRef - Sr M, C K, K K, et al. Understanding the molecular mechanisms for the enhanced phytoremediation of heavy metals through plant growth promoting rhizobacteria: A review. J Environ Manage. 2020;254. doi:10.1016/j.jenvman.2019.109779
CrossRef - de Souza R, Ambrosini A, Passaglia LMP. Plant growth-promoting bacteria as inoculants in agricultural soils. Genet Mol Biol. 2015;38(4):401-419. doi:10.1590/S1415-475738420150053
CrossRef - Ma Y, Rajkumar M, Zhang C, Freitas H. Beneficial role of bacterial endophytes in heavy metal phytoremediation. J Environ Manage. 2016;174:14-25. doi:10.1016/j.jenvman.2016.02.047
CrossRef - Z?och M, Thiem D, Gadza?a-Kopciuch R, Hrynkiewicz K. Synthesis of siderophores by plant-associated metallotolerant bacteria under exposure to Cd2+. Chemosphere. 2016;156:312-325. doi:10.1016/j.chemosphere.2016.04.130
CrossRef - Khan A, Singh P, Srivastava A. Synthesis, nature and utility of universal iron chelator – Siderophore: A review. Microbiol Res. 2018;212-213:103-111. doi:10.1016/j.micres.2017.10.012
CrossRef - da Costa PB, Granada CE, Ambrosini A, et al. A Model to Explain Plant Growth Promotion Traits: A Multivariate Analysis of 2,211 Bacterial Isolates. PLoS ONE. 2014;9(12):e116020. doi:10.1371/journal.pone.0116020
CrossRef - de Souza R, Beneduzi A, Ambrosini A, et al. The effect of plant growth-promoting rhizobacteria on the growth of rice (Oryza sativa L.) cropped in southern Brazilian fields. Plant Soil. 2013;366(1):585-603. doi:10.1007/s11104-012-1430-1
CrossRef - El-Meihy RM, Abou-Aly HE, Youssef AM, Tewfike TA, El-Alkshar EA. Efficiency of heavy metals-tolerant plant growth promoting bacteria for alleviating heavy metals toxicity on sorghum. Environ Exp Bot. 2019;162:295-301. doi:10.1016/j.envexpbot.2019.03.005
CrossRef - Bruno LB, Karthik C, Ma Y, Kadirvelu K, Freitas H, Rajkumar M. Amelioration of chromium and heat stresses in Sorghum bicolor by Cr6+ reducing-thermotolerant plant growth promoting bacteria. Chemosphere. 2020;244:125521. doi:10.1016/j.chemosphere.2019.125521
CrossRef - Jain D, Kour R, Bhojiya AA, et al. Zinc tolerant plant growth promoting bacteria alleviates phytotoxic effects of zinc on maize through zinc immobilization. Sci Rep. 2020;10(1):13865. doi:10.1038/s41598-020-70846-w
CrossRef - You LX, Zhang RR, Dai JX, et al. Potential of cadmium resistant Burkholderia contaminans strain ZCC in promoting growth of soy beans in the presence of cadmium. Ecotoxicol Environ Saf. 2021;211:111914. doi:10.1016/j.ecoenv.2021.111914
CrossRef - Wu Y, Ma L, Liu Q, et al. The plant-growth promoting bacteria promote cadmium uptake by inducing a hormonal crosstalk and lateral root formation in a hyperaccumulator plant Sedum alfredii. J Hazard Mater. 2020;395:122661. doi:10.1016/j.jhazmat.2020.122661
CrossRef - Banik A, Pandya P, Patel B, Rathod C, Dangar M. Characterization of halotolerant, pigmented, plant growth promoting bacteria of groundnut rhizosphere and its in-vitro evaluation of plant-microbe protocooperation to withstand salinity and metal stress. Sci Total Environ. 2018;630:231-242. doi:10.1016/j.scitotenv.2018.02.227
CrossRef - Ma Y, Rajkumar M, Freitas H. Improvement of plant growth and nickel uptake by nickel resistant-plant-growth promoting bacteria. J Hazard Mater. 2009;166(2-3):1154-1161. doi:10.1016/j.jhazmat.2008.12.018
CrossRef - Dary M, Chamber-Pérez MA, Palomares AJ, Pajuelo E. “In situ” phytostabilisation of heavy metal polluted soils using Lupinus luteus inoculated with metal resistant plant-growth promoting rhizobacteria. J Hazard Mater. 2010;177(1-3):323-330. doi:10.1016/j.jhazmat.2009.12.035
CrossRef - Huang H, Zhao Y, Fan L, Jin Q, Yang G, Xu Z. Improvement of manganese phytoremediation by Broussonetia papyrifera with two plant growth promoting (PGP) Bacillus species. Chemosphere. 2020;260:127614. doi:10.1016/j.chemosphere.2020.127614
CrossRef - Mallick I, Bhattacharyya C, Mukherji S, et al. Effective rhizoinoculation and biofilm formation by arsenic immobilizing halophilic plant growth promoting bacteria (PGPB) isolated from mangrove rhizosphere: A step towards arsenic rhizoremediation. Sci Total Environ. 2018;610-611:1239-1250. doi:10.1016/j.scitotenv.2017.07.234
CrossRef - Peng W, Li X, Song J, Jiang W, Liu Y, Fan W. Bioremediation of cadmium- and zinc-contaminated soil using Rhodobacter sphaeroides. Chemosphere. 2018;197:33-41. doi:10.1016/j.chemosphere.2018.01.017
CrossRef - Ren XM, Guo SJ, Tian W, et al. Effects of Plant Growth-Promoting Bacteria (PGPB) Inoculation on the Growth, Antioxidant Activity, Cu Uptake, and Bacterial Community Structure of Rape (Brassica napus L.) Grown in Cu-Contaminated Agricultural Soil. Front Microbiol. 2019;10. Accessed March 24, 2023. https://www.frontiersin.org/articles/10.3389/fmicb.2019.01455
CrossRef - Tanwir K, Javed MT, Abbas S, et al. Serratia sp. CP-13 alleviates Cd toxicity by morpho-physio-biochemical improvements, antioxidative potential and diminished Cd uptake in Zea mays L. cultivars differing in Cd tolerance. Ecotoxicol Environ Saf. 2021;208:111584. doi:10.1016/j.ecoenv.2020.111584
CrossRef - Rizvi A, Khan MS. Biotoxic impact of heavy metals on growth, oxidative stress and morphological changes in root structure of wheat (Triticum aestivum L.) and stress alleviation by Pseudomonas aeruginosa strain CPSB1. Chemosphere. 2017;185:942-952. doi:10.1016/j.chemosphere.2017.07.088
CrossRef - Ali A, Guo D, Li Y, et al. Streptomyces pactum addition to contaminated mining soils improved soil quality and enhanced metals phytoextraction by wheat in a green remediation trial. Chemosphere. 2021;273:129692. doi:10.1016/j.chemosphere.2021.129692
CrossRef - Syed A, Elgorban AM, Bahkali AH, Eswaramoorthy R, Iqbal RK, Danish S. Metal-tolerant and siderophore producing Pseudomonas fluorescence and Trichoderma spp. improved the growth, biochemical features and yield attributes of chickpea by lowering Cd uptake. Sci Rep. 2023;13(1):1-17. doi:10.1038/s41598-023-31330-3
CrossRef - Taniguchi J, Hemmi H, Tanahashi K, Amano N, Nakayama T, Nishino T. Zinc biosorption by a zinc-resistant bacterium, Brevibacterium sp. strain HZM-1. Appl Microbiol Biotechnol. 2000;54(4):581-588. doi:10.1007/s002530000415
CrossRef






