Diversity of Aquatic Macrophyte Species of Pardi Wetland, Valsad District, Gujarat, India: Social-economic and Ethnobotanical importance
1
Department of Botany,
Shrimad Rajchandra Vidyapeeth,
Dharampur, Valsad,
Gujarat
India
2
Department of Biological and Environmental Sciences,
Natubhai V. Patel College of Pure and Applied Sciences (NVPAS),
Vallabh Vidyanagar, Anand,
Gujarat
India
3
Department of Botany,
B.K.M Science college,
Tithal Road, Valsad,
Gujarat
India
Corresponding author Email: harviipatel2405@gmail.com
DOI: http://dx.doi.org/10.12944/CWE.18.3.32
Copy the following to cite this article:
Patel H. A, Sahoo S. Thakor A. Diversity of Aquatic Macrophyte Species of Pardi Wetland, Valsad District, Gujarat, India: Social-economic and Ethnobotanical importance. Curr World Environ 2023;18(3). DOI:http://dx.doi.org/10.12944/CWE.18.3.32
Copy the following to cite this URL:
Patel H. A, Sahoo S. Thakor A. Diversity of Aquatic Macrophyte Species of Pardi Wetland, Valsad District, Gujarat, India: Social-economic and Ethnobotanical importance. Curr World Environ 2023;18(3).
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2022-12-22 |
|---|---|
| Accepted: | 2023-06-14 |
| Reviewed by: | 
 Shemaa Fatih
Shemaa Fatih
|
| Second Review by: |

 Sadar Aslam
Sadar Aslam
|
| Final Approval by: | Dr. Igor M. Danilin |
Introduction
Water defines or impacts most, if not all, of a field’s biogeochemistry, or the biological, chemical, and physical features of a particular location, particularly areas of our terrain known as wetlands 1. Wetlands serve as the interface and ecotone habitat between the terrestrial ecosystem and the aquatic ecosystem. It indicates that wetlands are neither entirely aquatic nor terrestrial; depending on seasonal fluctuation, they may be both at a similar moment, which contains the water and aquatic life, two essential components of the ecosystem 2. Wetlands are geologically young and ecologically delicate; they exist in all climates and vary sporadically with the passage of time and season. The Earth has 5.3 to 12.8 million km2 of wetland area 3. Wetlands are natural water bodies, that support a huge number of floral and faunal diversity of the earth. Due to the diverse geographical location, dominant species, their genesis, water chemistry, and sediment or soil characteristics; wetlands express rich biodiversity 1. Globally, wetlands are a highly adaptive, productive, and biologically rich ecosystem that provides many significant private and public services to society with both consumptive and non-consumptive benefits. India supports a broad range of wetland ecosystems, with its varied terrain and climate regimes. In addition, there are 555,557 wetlands (with inland wetlands accounting for 69%, coastal wetlands for 27%, and other wetlands (less than 2.25 ha) accounting for 4% of total wetlands 5.Wetland has currently covered 15.26 million hectares (mh), or 4.63 % land area of India. In terms of total wetland area covered by the state (in percentage), represent that Gujarat state ranks first with 3.47 million hectares (22.77%), mainly because of the large stretches of salt pans and intertidal mudflats.
The aquatic environment generally consists of a large range of floral and faunal diversity, which increasingly serves human requirements through contributions to agriculture, biofertilizers, sources of energy, raw materials for industry, and medicines 2. Natural freshwater resources found across the world frequently provide an ideal habitat for the colonization of various macrophyte species 3. Herbal macrophytes have been used as traditional medicine since ancient times because they are amazing sources of physiologically active chemicals with medicinal qualities 9. Although herbal macrophytes play a significant role in disease prevention, and treatment and have also been used to cure a variety of human ailments by various groups of people 10, 11. There are few ethnobotanical records, particularly on the medicinal macrophytes found in the Valsad area. The majority of regional communities live in various sections of the state and rely on their collective expertise and local resources for their daily healthcare. Due to a variety of factors, older generations are the only ones who are generally knowledgeable about plant lore, its significance, and its practice 4–6. In the current communication, the documentation of aquatic medicinal plants found in the wetlands of the Valsad district is discussed. It is widely known that worldwide wetlands are disappearing fast and thus their resources both flora and fauna are depleting at the same pace7. The survival of aquatic species is threatened 8, 9, and hence the study on aquatic resources especially those having economic value are important. 10
Study area
Gujarat's southernmost district, Valsad, is situated on the Arabian Sea's shore, the global position is located at 20°37'48.00" North latitude and 72°55'48.00" East longitude with an average elevation of 42 feet (13 meters) 5. The district covers a 3034 sq. km geographic area and a 23116-ha wetland area, which are 0.67 % of the total wetland area 5. Pardi wetland is an important lake of the Valsad district, situated at Pardi village near the Pardi Gujarat Industrial Development Corporation (GIDC) (Fig: 1). This perennial lake is located at 20o 30’ 35.7” N 72o 57’ 11.9” E coordinates and has a perimeter of 2298.25 m. The depth of this lake has continuously fluctuated during the study period due to the Sujlam Suflam Yojna. The average depth of the lake was 6 to 7 meters during the monsoon season and reduced to 1 to 2 meters during the summer season, but it did not dry out and the lake stored a high amount of water. This lake carries a considerable volume of household sewage, industrial run-off as well as a huge amount of agricultural runoff from the surrounding area 11.
 | Figure 1: Selected study area of Valsad district, Gujarat.
|
Materials and methods
Throughout the study period (January 2019 to December 2019), field visits were made to collect and precisely record the aquatic macrophyte species that were present at the Pardi wetland in Valsad district, Gujarat, India. From January 2019 to December 2019, the survey was carried out every three months, covering all seasons (winter, summer, monsoon), however, in order to have a better knowledge of seasonal fluctuation, we collected specimens, initially as well as at the end of the month, during winter (January month), summer (April month), pre-monsoon (July month), and post-monsoon (October month) seasons. For the flora study, the quadrate method (50 cm 2) was randomly applied in 20 different places and analysed. 12 The collected macrophyte species were identified with the guidance of the herbarium, Botany department, VNSGU, and floras 12, 13. The macrophyte species were classified into six subcategories based on their growth form, including free-floating, floating but rooted, submerged but not rooted, submerged but rooted amphibious macrophytes, and emergent macrophytes, prescribed by Pedralli, (1990) methods.12 The life form classification study was done as per Raunkiaer's (1934) life form classification system as modified by the researcher Ellenberg and Mueller-Dombois (1967), and Mueller-Dombois and Ellenberg (1974).12–15
Personal interviews and conversations with local people especially with senior villagers were used to gather socio-economic and ethnobotanical data by interviewing through a questionnaire 45 local informers, including 25 females and 20 males. During the research period, regular fieldwork and survey were organized in the different surrounding villages of the selected wetland, Pardi Lake. Data were gathered on different aspects by asking questions about the aquatic plant, its local names, used parts and how these parts were utilized, and for which diseases treatment. The interviewer was asked questions in Gujarati because of the ease of local inhabitants who are uneducated and the English language is not logical in most cases. A list of diverse economically relevant macrophytes base of uses was compiled because locals utilize macrophytes in a variety of ways, the research involves personal interviews, observations, and surveys. These folks are the only ones who know the names of the indigenous macrophyte species and how they may be used in different ways. Many questionnaires were utilized during these activities, such as the usage of macrophytes, which plant parts were used, economic worth, and so on.
Result
Anyone can simply obtain information about a site’s species diversity by looking at its floristic composition list. According to Gupta (2017), one of the important anatomical characteristics of the plant community is an area's floristic composition.16 It has been documented 43 macrophyte species at the Pardi wetland of the Valsad district, Gujarat (Table: 1). At the selected wetland, Pardi Lake in Gujarat's Valsad district, 43 species of macrophytes from 35 genera and 24 families were documented during the investigation (Table: 1). Out of the 43 macrophyte species, 40 macrophyte species represented angiosperms, two macrophyte species like as Azolla pinnata R. Br., and Marselia quadrifolia L. represented the pteridophytes, and one species of macrophyte, Chara globularis L. represented macroalgae (Table: 1).
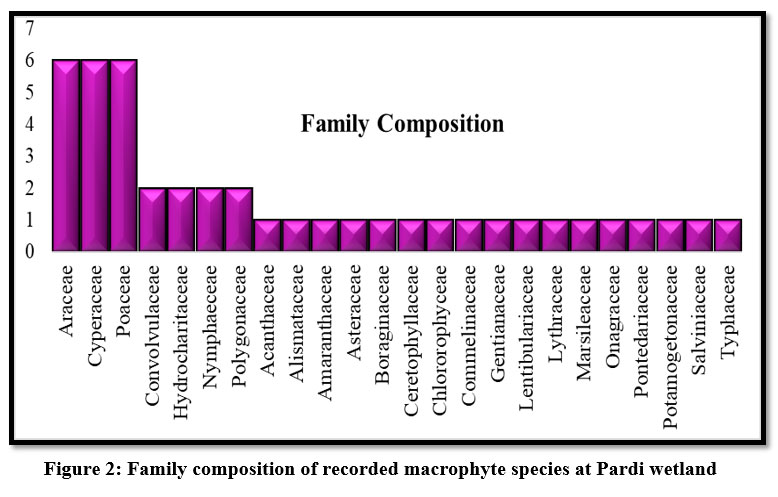 | Figure 2: Family composition of recorded macrophyte species at Pardi wetland.
|
Among the total family, Araceae, Cyperaceae, and Poaceae was the largest one which comprises six macrophyte species followed by Convolvulaceae, Hydrocharitaceae, Nymphaeceae, and Polygonaceae (two macrophyte species), and others were considered as monospecific ones (Fig: 2).
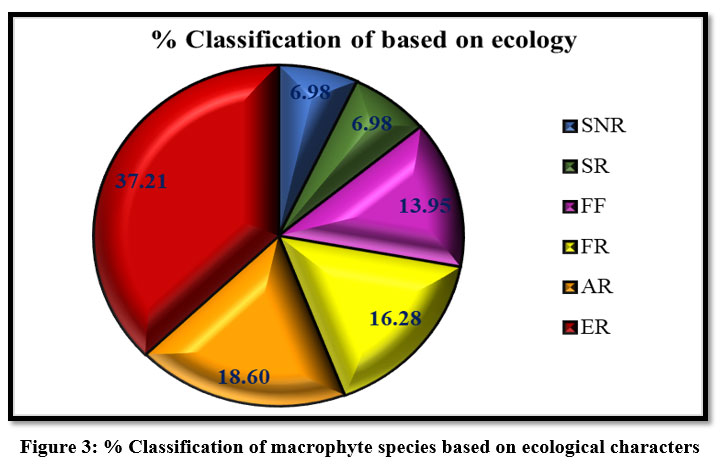 | Figure 3: % Classification of macrophyte species based on ecological characters.
|
The aquatic species were classified based on growth forms 13, into six major categories i) free-floating, ii) floating but rooted, iii) submerged but rooted, iv) submerged but not rooted, v) amphibious and rooted; and vi) emergent macrophytes (Table: 1). Out of 43 macrophyte species, 6.98 % macrophytes represents submerged but rooted group, and 6.98 % are under submerged but not rooted group. Submerged species are vascular, and their vegetative development occurs below the water's surface. With increasing water depth or solid particles suspended in water, light intensity drops, due to this reason, they contain less macrophyte diversity 18,19. A total of 13.95 % were under the free-floating category, 16.28 % of macrophytes were under the rooted with floating leaves category, and 18.60 % of macrophyte species represent the amphibious category. Emergent macrophytes are the most common kind of aquatic vegetation, out-competing other types due to their capacity to catch sunlight before it reaches the water's surface 20,21, due to these reasons emergent macrophytes are dominant with 37.21 %, over the process of the investigation (Fig: 3).
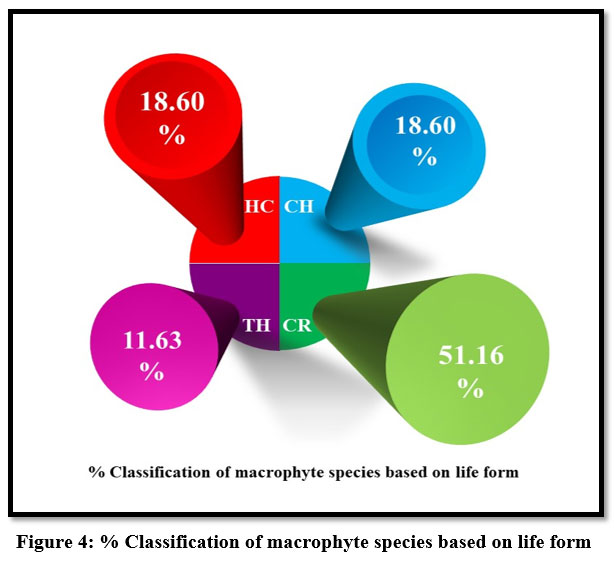 | Figure 4: % Classification of macrophyte species based on life form
|
The composition of vegetation’s life forms is an essential foundation for any ecological research. The distribution of a community’s life forms provides information on the community’s reaction to specific environmental variables. 13-15 The Raunkiaer’s one, according to Singh and Gupta (2015), is the best-recognized description and classification of life forms, as well as the utilization of life forms to create a biological spectrum.21 Raunkiaer grouped species’ life forms in natural succession, using the primary criterion of the location of perennating buds, and he demonstrated that this criterion was accepted to indicate climatic adaptability 15. The macrophytes in this study were categorized into different life forms using Raunkiaer’s categorization. Figure: 4 shows the various life-form categories, macrophyte species within these groups, and their percentages of occurrence. Cryptophytes have the highest percentage of them (51.16 %), followed by Chamophytes (18.60 %), Hemi cryptophytes (18.60 %), and Therophytes (11.63 %).
A list of plants found in wetlands with their associated indicator statuses can be found on the national list of plant species 23. The five indicator statuses are Obligate Wetland Plants (OBL), Facultative Wetland Plants (FACW), Facultative Plants (FAC), Facultative Upland Plants (FACU), Obligate Upland Plants (UPL). Based on these indicator statuses total of 43 species of macrophytes were categorized and represented in Table 1. Out of these 43 macrophyte species, 46.51 % macrophyte species belong to Facultative Wetland Plants, 37.21 % species belong to Obligate Upland Plants, 9.30 % species belong to Facultative Plants, 4.65 % macrophyte species belong to Facultative Upland Plants and 2.33 % macrophytes represent the Obligate Upland Plants (Fig: 5).
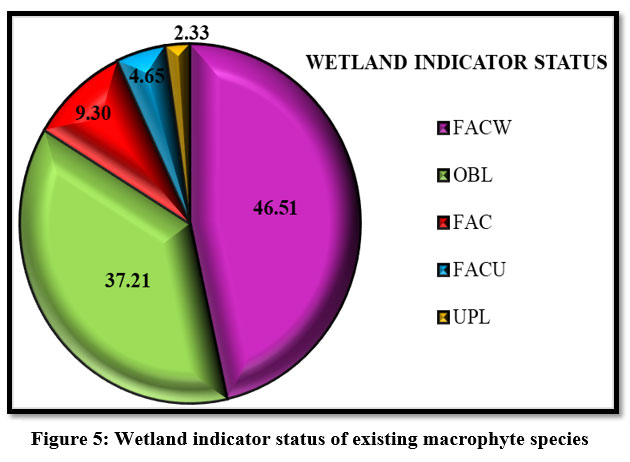 | Figure 5: Wetland indicator status of existing macrophyte species.
|
During fieldwork at Pardi wetland total of 43 macrophyte species were identified and listed based on the visual observation in the quadrats by using Shah (1978) and Cook (1908). 23-24 The given tables show the list of aquatic macrophytes with their indicator statuses, ecological type, local name, family, and life form classification (Table: 1), and socioeconomic and ethnobotanical importance of existing macrophyte species (Table: 2).
Table 1: Floristic composition of present macrophyte species at Pardi wetland.
No | Scientific name | Local name | Type | Life form | Indicator status |
| Acanthaceae |
|
| |||
1 | Hygrophila auriculata (Schum.) Heine | Aakhiryo | ER | TH | FAC |
| Alismataceae |
|
| |||
2 | Limnophyton obtusifolium (L.) Miq |
| FR | HC | FACW |
| Amaranthaceae |
|
| |||
3 | Alternanthera sessilis (L.) Dc. | Fulyu | ER | TH | FAC |
| Araceae |
|
| |||
4 | Colocasia esculenta (L.) Schott | Toro/Jungli Patra | AR | CR | FACW |
5 | Lemna minor L. |
| FF | CR | OBL |
6 | Lemna trisulca L. |
| FF | CR | OBL |
7 | Pistia stratiotes L. | Jal srunkhla | FF | CR | OBL |
8 | Spirodela polyrhiza (L.) Schleid. |
| FF | CR | OBL |
9 | Wolffia arrhiza (L.) Wimmer |
| SNR | CR | OBL |
| Asteraceae |
|
| |||
10 | Grangea maderaspatana (L.) Poir. |
| ER | HC | FACU |
| Boraginaceae |
|
| |||
11 | Coldenia procumbens L. | Basario okharad | ER | HC | FACU |
| Ceretophyllaceae |
|
| |||
12 | Ceratophyllum demersum L. |
| SNR | CR | OBL |
| Chlororophyceae |
|
| |||
13 | Chara globularis L. |
| SNR | CR | OBL |
| Commelinaceae |
|
| |||
14 | Commelina benghalensis L. | Motun sismulyun | AR | HC | FAC |
| Convolvulaceae |
|
| |||
15 | Ipomoea aquatica Forsk. | Nari bhji | FR | HC | FACW |
16 | Ipomoea fistulosa Mart. ex Choisy | Naravel | ER | CH | UPL |
| Cyperaceae |
|
| |||
17 | Cyperus articulatus L. |
| ER | CR | FACW |
18 | Cyperus iria L. |
| ER | CR | FACW |
19 | Cyperus pumilus L. |
| ER | CR | FACW |
20 | Cyperus triceps (Rottb.) Endl. |
| ER | CR | FACW |
21 | Eleocharis acutangula (Roxb.) Schult. |
| AR | CH | FACW |
22 | Eleocharis dulcis (Burm. f.) Henschel. |
| AR | CH | FACW |
| Gentianaceae |
|
| |||
23 | Nymphoides indicum (L.) O. Ktze. |
| FR | CR | OBL |
| Hydrocharitaceae |
|
| |||
24 | Hydrilla verticillata (L. f.) Royle |
| SR | CR | OBL |
25 | Vallisneria spiralis L. | Jal sarpoliya | SR | CH | OBL |
| Lentibulariaceae |
|
| |||
26 | Utricularia aurea Lour. |
| SR | CR | OBL |
| Lythraceae |
|
| |||
27 | Ammania multiflora Roxb. |
| ER | TH | FAC |
| Marsileaceae |
|
| |||
28 | Marselia quadrifolia L. | Swastik Patri | AR | CR | FACW |
| Nymphaeceae |
|
| |||
29 | Nelumbo nucifera Gaertn. | Kamal | FR | TH | OBL |
30 | Nymphaea nouchali Willd | Poyna | FR | CR | OBL |
| Onagraceae |
|
| |||
31 | Ludwigia adscendens L. | Talav bhaji | FR | CR | FACW |
| Poaceae |
|
| |||
32 | Chloris quinquesetica Bhide |
| ER | HC | FACW |
33 | Dactyloctenium aegyptium (L.) P. Beauv. | Darbha | ER | CH | FACW |
34 | Echinochloa crusgalli (L.) P. Beauv. |
| ER | CH | FACW |
35 | Eleusine indica (L.) Geaertn. |
| ER | TH | FACW |
36 | Eragrostis tenella (L.) |
| ER | TH | FACW |
37 | Eragrostis unioloides (Retz.) Nees ex Steud. |
| ER | HC | FACW |
| Polygonaceae |
|
| |||
38 | Polygonum barbatum L. |
| AR | TH | FACW |
39 | Polygonum glabrum Willd |
| AR | CH | FACW |
| Pontedariaceae |
|
| |||
40 | Eichhornia crassipes (Mart.) Solms. | Jal kumbhi | FF | CH | OBL |
| Potamogetonaceae |
|
| |||
41 | Potamogeton nodosus Poir. |
| FR | CR | OBL |
| Salviniaceae |
|
| |||
42 | Azolla pinnata R. Br. |
| FF | TH | OBL |
| Typhaceae |
|
| |||
43 | Typha angustata Bory & Chaub. | Gha bajri | AR | CR | FACW |
(Abbreviations used in the table: FF = Free floating, FR = Floating but rooted, SNR = Submerged but not rooted, SR = Submerged but rooted, ER = Emergent, CH = Chamophytes, HC = Hemicryptophytes, CR = Cryptophytes, TH = Therophytes, OBL = Obligate Wetland Plants, FACW = Facultative Wetland Plants, FAC = Facultative Plants, FACU = Facultative Upland Plants, UPL = Obligate Upland Plants).
Table 2: Socio-economic and ethnobotanical importance of macrophyte species at Pardi wetland
Species | Part(s) used | Uses |
A. sessilis (L.) Dc. | Whole macrophyte |
|
A. multiflora Roxb.
| Leaf
|
|
A. pinnata R. Br.
| Whole macrophyte |
|
C. demersum L. | Whole macrophyte |
|
C. procumbens L.
| Leaves
|
|
C. esculenta (L.) Schott
| Leaves, petiole, & corm |
|
C. benghalensis L. | Leaves & young shoots |
|
C. iria L. | Stems |
|
E. crassipes (Mart.) Solms. | Whole macrophyte |
|
E. dulcis (Burm. f.) Henschel. | Whole macrophyte |
|
H. verticillata (L. f.) Royle | Whole macrophyte |
|
H. auriculata (Schum.) Heine | Leaves
|
|
I. aquatica Forsk. | Young tender shoots & leaves |
|
I. fistulosa Mart. ex Choisy | Whole macrophyte |
|
L. minor L. | Whole macrophyte
|
|
L. trisulca L.
| Whole macrophyte |
|
L. adscendens L.
| Young tender shoots & leaves
|
|
M. quadrifolia L. | Leaves & twig |
|
N. nucifera Gaertn. & N. nouchali Willd | Whole macrophyte
|
|
P. stratiotes L.
| Whole macrophyte |
|
S. polyrhiza Linn. | Whole macrophyte |
|
T. angustata Bory & Chaub.
| Whole macrophyte
|
|
V. spiralis L. | Leaves |
|
Discussion
The selected site represents less microphyte diversity because of the huge anthropogenic pressure and industrial effluents, besides some species are dominant at Pardi Lake because of the favourable environment. A similar observation was found by 26 in Gala Lake, Turkey.
The majority of morphologically submerged macrophyte species are vascular, and their vegetative development occurs below the water’s surface 27. With increasing water depth or solid suspended particles in water, light intensity drops, due to this reason they are so weak. 27 This group represented the lowest floristic percentage over the process of the investigation (6.98 %) (Fig: 2). The root system, rooted with a floating group of macrophytes, is poorly developed, plant growth is reduced due to low light intensity, stems are generally long and slender, leaves have recurred and thin, narrow, ribbon-shaped, and finely dissected, stems are generally long and slender, stems are generally long and slender 28. The free-floating macrophytes, on the other hand, float freely on the water's surface. The shooting system isn't very well developed. Internodes are extremely short and compact. The root system is underdeveloped and lacks root hairs and the leaves are broad. 29 The majority of the amphibious macrophytes have rhizomes and have two types of leaves, submerged leaves which are narrow and heavily dissected, whereas aerial leaves are broad or just slightly lobed, with a propensity to increase the exposed surface area 30. Emergent macrophytes are the most common kind of aquatic vegetation 31, out-competing other types due to their capacity to catch sunlight before it reaches the water's surface 32, due to these reasons emergent macrophytes are dominant with 37.21 %, over the process of the investigation (Fig: 2). A similar observation was obtained by (Malik & Namdeo, 2010) in the polluted pond of Shahjahanpur (India); 34 in the wetlands of Hojai subdivision, Nagaon district, Assam, India.
The region may be classified as a cryptophytic climate based on these observations of the percentage composition of various life form classes of macrophytes at the research site. Cryptophytic condition indicates a warm and dry climate 35. The existence of heavy anthropogenic activity in the wetlands, where low percentages of Hemicryptophytes showed unsuitability of the environment, may explain the suggestion of warm and dry conditions by exhibiting a high percentage of occurrence of cryptophytes. 36 Macrophyte species are general markers of varying degrees of environmental conditions; nevertheless, they are not accurate 37. The existence of a macrophyte species at a location is determined by many climatic, edaphic, and biotic conditions, and it is hard to separate the influence of specific elements such as degree of substrate saturation and depth and duration of standing water.38
Conclusion
Anthropogenic activities have impacted wetland ecosystem services across the world. It is well-known that global wetlands are shrinking rapidly and hence their resources both floral and faunal are depleting at the same pace. In the developing world restoration of wetlands is highly needed. The present study shows the macrophyte species composition in the wetland ecosystem and the socio-economic benefit of the present macrophyte species. The survival of aquatic species is threatened and hence the study of aquatic resources especially those having economic value are important. The production of wetlands macrophytes is excessive, which benefits the rural as well as urban economy. Many macrophytes are harvested by underprivileged people living in nearby wetland regions, such as neighbouring villages, and sold in local marketplaces for daily use as food, fodder, thatching, medicine, and other reasons. The study will also help to recreate wetland diversity precisely to Valsad District which has the maximum number of wetlands in Gujarat state. Furthermore, the study can be also helpful for understanding the complexity of the wetland ecosystem. Besides, Wetlands not only provide useful resources but are also important in terms of ecology and maintaining the climate of the region. Therefore, the conservation of wetlands especially in Valsad district, Gujarat needs to be addressed urgently.
Acknowledgment
Authors are thankful to Dr. R. M. Patel, as well as the Botany department, VNSGU (Veer Narmad South Gujarat University) for the identification of collected specimens, and also thank the local people who help us for the socio-economic and ethnobotanical knowledge.
Conflict of Interest
The authors do not have any conflict of interest.
Funding Sources
The authors appreciate the financial support provided by UGC New Delhi –NFOBC (Candidate ID: NFO-2018-19- OBC-GUJ-66980).
References
- Barbier EB. Les zones humides en tant que biens naturels. Hydrol Sci J. 2011;56(8):1360-1373. doi:10.1080/02626667.2011.629787
CrossRef - FAO. Aquatic Plants Their Uses and Risks.; 2012.
- Cantonati M, Poikane S, Pringle CM, et al. Natural and Artificial Freshwater Environments?: Consequences for Biodiversity Conservation. Water. 2020;12(November 2019):260.
CrossRef - Jain A, Roshnibala S, Kanjilal PB, Singh RS, Singh B. Aquatic/semi-aquatic plants used in herbal remedies in the wetlands of Manipur, Northeastern India. Indian J Tradit Knowl. 2007;6(2):346-351.
- Ali M, Iqbal IM, Shabbir A, Khan Z ud din. Ethnomedicinal studies on aquatic plants of tehsil. 2020;8(1):15-19.
CrossRef - Ya VB. Aquatic plants of the Far East of Russia: A review on their use in medicine, pharmacological activity. Bangladesh J Med Sci. 2015;14(1):9-13. doi:10.3329/bjms.v14i1.21554
CrossRef - Tsukamoto Y, Yonezawa S, Katayama N, Isagi Y. Detection of Endangered Aquatic Plants in Rapid Streams Using Environmental DNA. Front Ecol Evol. 2021;8(January):1-7. doi:10.3389/fevo.2020.622291
CrossRef - Nuamah LA, Li Y, Pu Y, et al. Constructed wetlands, status, progress, and challenges. The need for critical operational reassessment for a cleaner productive ecosystem. J Clean Prod. 2020;269:122340. doi:10.1016/j.jclepro.2020.122340
CrossRef - Chatterjee K, Bandyopadhyay A, Ghosh A, Kar S. Assessment of environmental factors causing wetland degradation, using Fuzzy Analytic Network Process: A case study on Keoladeo National Park, India. Ecol Modell. 2015;316:1-13. doi:10.1016/j.ecolmodel.2015.07.029
CrossRef - O’Hare MT, Aguiar FC, Asaeda T, et al. Plants in aquatic ecosystems: current trends and future directions. Hydrobiologia. 2018;812(1). doi:10.1007/s10750-017-3190-7
CrossRef - Patel H, S Sahoo. A Floristic Account of Macrophytes in the Selected Wetlands of Valsad District, Gujarat, India. Int J Lakes Rivers. 2021;14(1):113-121.
https://www.ripublication.com/ijlr21/ijlrv14n1_08.pdf - Odum EP. Fundamentals of Ecology. Third Edit. (Co WBS, ed.).; 1971.
- Pedralli G. Macrófitos aquáticos: técnicas e métodos de estudos. Estud Biol. 1990;26:5-24.
- Raunkiær, C. C., Fausbøll, A. I., Gilvert-Carter, H., & Tansley AGS. The Life Forms of Plants and Statistical Plant Geography: Being the Collected Papers of C. Raunkiaer. Oxford, UK: Clarendon Press.; 1934.
- Ellenberg, H. and Mueller-Dombois D. A key to Raunkiaer plant life- forms with revised subdivisions. Berichte des Geobot Institutes der Eidg Techn Hochschule, Stift Rübel. 1967;37:56-73.
- Mueller-Dombois, D. and Ellenberg H. Aims and Methods of Vegetation Ecology. John Wiley and Sons,; 1974.
- Gupta A. FLORISTIC DISTRIBUTION AND GROWTH FORM ANALYSIS OF MACROPHYTES IN KONGBA RIVER , MANIPUR. 2017;(January 2009).
- Hilt S, Alirangues Nuñez MM, Bakker ES, et al. Response of submerged macrophyte communities to external and internal restoration measures in north temperate shallow lakes. Front Plant Sci. 2018;9(February). doi:10.3389/fpls.2018.00194
CrossRef - Liu L, Bu XQ, Wan JY, et al. Impacts of sediment type on the performance and composition of submerged macrophyte communities. Aquat Ecol. 2017;51(1):167-176. doi:10.1007/s10452-016-9607-y
CrossRef - Li S, Sun T, Yang W, Cui B, Yin X. A biodiversity evaluation framework for restoration of aquatic macrophyte communities in shallow lakes driven by hydrological process management. Hydrol Process. 2021;35(1):1-16. doi:10.1002/hyp.13983
CrossRef - Zhang Y, Ma R, Liang Q, Guan B, Loiselle S. Secondary impacts of eutrophication control activities in shallow lakes: Lessons from aquatic macrophyte dynamics in lake taihu from 2000 to 2015. Freshw Sci. 2019;38(4):802-817. doi:10.1086/706197
CrossRef - Singh RKI, Gupta A. Life-form classification and biological spectrum of Amambilok Sacred Grove , Andro , Manipur in Northeast India. 2015;9(2):356-364.
- McCoy-Sulentic ME, Kolb TE, Merritt DM, Palmquist EC, Ralston BE, Sarr DA. Variation in species-level plant functional traits over wetland indicator status categories. Ecol Evol. 2017;7(11):3732-3744. doi:10.1002/ece3.2975
CrossRef - Shah GL. Flora of Gujarat State. Vol. I & II. Sardar Patel University, Press, Vallabh Vidhyanagar,; 1978.
CrossRef - Cook. T. The Flora of the Presidency of Bombay. Taylor & Francis; London; 1908.
- Efe R, Bizzarri C, Curebal I, Nyusupova G. Environment and Ecology at the Beginning of 21 St Century Editors.; 2015.
- Poschenrieder C, Fernández JA, Rubio L, Pérez L, Terés J, Barceló J. Transport and use of bicarbonate in plants: Current knowledge and challenges ahead. Int J Mol Sci. 2018;19(5):1-25. doi:10.3390/ijms19051352
CrossRef - Ebke KP, Felten C, Dören L. Impact of heterophylly on the sensitivity of Myriophyllum aquaticum biotests. Environ Sci Eur. 2013;25(1):1-9. doi:10.1186/2190-4715-25-6
CrossRef - Ceschin S, Ferrante G, Mariani F, Traversetti L, Ellwood NTW. Habitat change and alteration of plant and invertebrate communities in waterbodies dominated by the invasive alien macrophyte Lemna minuta Kunth. Biol Invasions. 2020;22(4):1325-1337. doi:10.1007/s10530-019-02185-5
CrossRef - Riis T, Olesen B, Clayton JS, Lambertini C, Brix H, Sorrell BK. Growth and morphology in relation to temperature and light availability during the establishment of three invasive aquatic plant species. Aquat Bot. 2012;102:56-64. doi:10.1016/j.aquabot.2012.05.002
CrossRef - Kassa Y, Mengistu S, Wondie A, Tibebe D. Distribution of macrophytes in relation to physico-chemical characters in the south western littoral zone of Lake Tana, Ethiopia. Aquat Bot. 2021;170(December 2020):103351. doi:10.1016/j.aquabot.2020.103351
CrossRef - Gebrehiwot M, Kifle D, Triest L. Emergent Macrophytes Support Zooplankton in a Shallow Tropical Lake: A Basis for Wetland Conservation. Environ Manage. 2017;60(6):1127-1138. doi:10.1007/s00267-017-0935-z
CrossRef - Malik SA, Namdeo A. Phytosociological Study of Macrophytes in a Polluted Pond of. 2010;1(5):1-3.
- Saikia M. Aquatic Macrophytes of the Wetlands of Hojai Sub Division , Nagaon District of Assam , India. 2013;2(3):141-145.
- Elmslie BG, Gushulak CAC, Boreux MP, Lamoureux SF, Leavitt PR, Cumming BF. Complex responses of phototrophic communities to climate warming during the Holocene of northeastern Ontario, Canada. Holocene. 2020;30(2):272-288. doi:10.1177/0959683619883014
CrossRef - Gushulak CAC, Leavitt PR, Cumming BF. Basin-specific records of lake oligotrophication during the middle-to-late Holocene in boreal northeast Ontario, Canada. Holocene. 2021;31(10):1539-1554. doi:10.1177/09596836211025972
CrossRef - Ervin GN, Herman BD, Bried JT, Christopher Holly D. Evaluating non-native species and wetland indicator status as components of wetlands floristic assessment. Wetlands. 2006;26(4):1114-1129. doi:10.1672/0277-5212(2006)26[1114:ENSAWI]2.0.CO;2
CrossRef






