Physico-Chemical Parameters and Diversity of Phytoplankton in Kirtankhola River, Bangladesh
1
Corresponding author Email: rhossen@bu.ac.bd
DOI: http://dx.doi.org/10.12944/CWE.16.1.19
Copy the following to cite this article:
Hossen R, Chakraborty S, Karmoker D, Das S. K. Physico-Chemical Parameters and Diversity of Phytoplankton in Kirtankhola River, Bangladesh. Curr World Environ 2021;16(1). DOI:http://dx.doi.org/10.12944/CWE.16.1.19
Copy the following to cite this URL:
Hossen R, Chakraborty S, Karmoker D, Das S. K. Physico-Chemical Parameters and Diversity of Phytoplankton in Kirtankhola River, Bangladesh. Curr World Environ 2021;16(1). Available From : https://bit.ly/2Ogd5rL
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2020-11-02 |
|---|---|
| Accepted: | 2021-01-29 |
| Reviewed by: | 
 Tessy Paul P.
Tessy Paul P.
|
| Second Review by: |

 L.K Prasad
L.K Prasad
|
| Final Approval by: | Dr. Marta Luciane Fischer |
Introduction
Bangladesh is a land of water, crisscrossed with almost 700 rivers. Numerous rivers flow through Barishal (administrative division), located in south-central part of Bangladesh and, Kirtankhola river is a notable and one of the major rivers in Barishal division. The total length of the river is about 160 km, but the 21 km of the river flowing through Barishal and Jhalokathi district under the Barishal division is actively known as Kirtankhola (Mahbub,1). The river Kirtankhola with the second largest river port in Bangladesh plays vital roles in lives and livelihoods of people living in the vicinity of the river, and also in the socio-economy of the region. The river is the significant gateway to this division as well as agricultural practices, industrial progresses and human settlements are very common in nearby lands. Thereby, the river would expose to heavy pollution due to direct discharge of oil and wastages from the water vehicles, agricultural runoffs, and dumping of industrial and municipal wastages. Consequently, its water quality is changing which severely would affect the aquatic biota of the river especially phytoplankton (Kumar and Dua, 2).
To assess water quality, it is crucial to measure the physical and chemical factors found in the concerning water bodies. Physico-chemical factors have direct effects on the growth of phytoplankton, the primary producers on which other life forms are directly or indirectly dependent in the aquatic ecosystem (Partensky,3). Numerous studies showed that the phytoplankton communities are sensitive to changes in their environment, thereby, can be used as an effective bio-indicator for assessing water quality along with assessing the physico-chemical properties (Reynolds,4; Brettum and Andersen,5). However, understanding the diversity or ecological status like species abundance, richness and evenness of phytoplankton in aquatic ecosystem is highly complex and ecologists use different indices to measure the status of any area (Maguran,6; Purvis and Hector,7). According to various studies, species richness and evenness of species can be measured by most widely used diversity indices i.e. Margalef index and Simpson index respectively, whereas Shannon diversity index is for knowing combined status of species abundance or richness and evenness (Shannon and Weiner,8; Spellerberg and Peter,9; Simpson,10).
In aquatic ecosystem, quantitative and qualitative abundance of the phytoplankton, and phytoplankton diversity which plays crucial role in increasing productivity, has been reported directly correlated with existing water quality conditions of the water body (Newall,11; Vallina,12). However, fluctuation in water physico-chemical properties can trigger luxurious growth of certain groups of phytoplankton which can cause degradation of surface water quality resulting into deoxygenation of the water leading to death of other organisms (Whitton and Patts,13). Therefore, the growth and development of phytoplankton communities in relation to the water physico-chemical factors, influencing the water quality has been researched over the last few decades (Akbay,14; Peerapornpisal,15; Elliott,16). Assessing the water physico-chemical factors supporting the phytoplankton growth, and interactions between the physical and chemical factors such as dissolved oxygen (DO), temperature, nitrogen and phosphorous, pH, electrical conductivity (EC) and salinity (TDS) are very complex (Goldman and Horne,17).
There are several reports available on phytoplankton communities from Barishal region (Islam and Alfasane,18; Khondker,19; Chakraborty,20) and Rajonee,21 reported the water physico-chemical parameters of Kirtankhola River, but no investigation has been done on phytoplankton diversity in relation to the water physico-chemical parameters of this river yet. Therefore, the present study aimed to measure the water physico-chemical parameters along with phytoplankton diversity to assess the correlations among the parameters and different phytoplankton groups in this river. Results of the study would be helpful in details studies on water quality, pollution and impact on biodiversity of the river in future.
Materials and Methods
Study Site
The research was conducted in Kirtankhola River at six sampling locations. Each of the locations (stations) located at approximately 2.5 km distance covering in total 13 km of the river and the average width of the site is 500 m. Details of the site is represented in Figure 1.
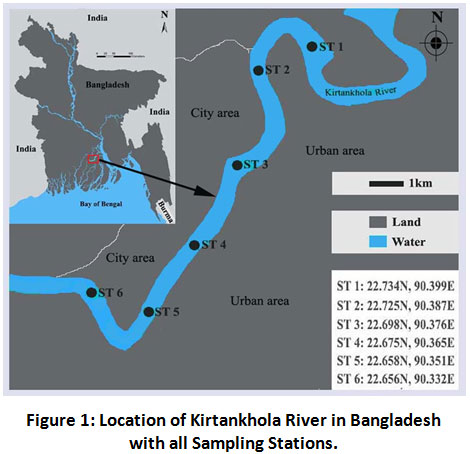 | Figure 1: Location of Kirtankhola River in Bangladesh with all Sampling Stations. Click here to view Figure |
Water Sampling
Water samples were collected from the surface to 40cm depth of water by Ruttner sampler and other sampling bottles during July 2020 to September 2020. Collections were completed between 7 to 10 am with the help of an engine boat. The water temperature, pH, TDS (Total dissolved solid), EC (Electrical conductivity) and DO (Dissolve oxygen) were measured using portable devices (Digital thermometer, HI98108 pHep+, HI98302 DiST2 and HI98304 DiST4) during the sampling time immediately after collections. To measure the rest of the physico-chemical parameters, water samples were taken in plastic containers and sealed with aluminum foil. Moreover, 1 L of sample water was transferred to graduated cylinders adding with few drops of Lugol’s solution and 4% neutral formalin for measuring phytoplankton diversity. Air temperature was in between 29 to 26.5 oC during the studied time.
Laboratory Analysis
NO3-, NH3, SO42- and CO3 2- were measured following standard methods (APHA,22). After 48 hours, 100 ml concentrated sediment was taken from the graduated cylinders to identify and count phytoplankton species using light microscope and heamocytometer. For identification confirmation, Bellinger and Sigee,23 Ahmed et al, 24 Islam and Moniruzzaman, 25 and Smith, 26 were consulted. The presented taxonomic arrangements were prepared following Komárek & Fott, 27 and Bold & Wynne, 28. MS excel was used to calculate the average and mean value of all the collected data. Then program PAST was applied to calculate the three diversity indices (Shannon, Simpson and Margalef index) where phytoplankton were expressed as organisms per ml. Another software program JMP was used to determine the correlations among the physico-chemical parameters and phytoplankton groups significant at 95% confidence level (P ? 0.05).
Results and Discussion
Physico-Chemical Parameters
From the investigated physico-chemical parameters average temperature was from 17.7 to 18.6 ÌŠC respectively in all stations. ST 6 looked different by most of the measured parameters, for example it gave the highest value in pH (8.45), EC (260 µS/cm), TDS (355 mg/l), NO3- (1.15 mg/l), NH3 (1.05 mg/l), DIP (4.10 mg/l) and lowest in CO3 2- (Table 1). These values concluded that the water of the station was comparatively more contaminated and it may due to the effluents from factories nearby bank of the station. On the other hand, ST 5 showed least amount in 5 parameters (DO, EC, TDS, NO3- and SO42-), that indicated the less contamination probably due to fewer anthropogenic impacts in this station area. ST 3 located next to the river port and provided highest concentration of DO, SO42- and CO3 2- and lowest pH, which meant water of this station is being acidic compared to the water samples from at other stations. Most probably, movements of water vehicles were the reasons for more dissolved oxygen and the wastage or oil discharge may be the reason for increase in SO42- and CO3 2- level. However, ST 1, 2 and 4 showed comparatively moderate values for maximum parameters that indicated similar environmental surroundings and less anthropogenic activities than ST 3 and 6. However, water of Kirtankhola was not polluted during the study time according to World Health Organization water quality guidelines (WHO, 29). But water of the area of ST 3 and ST 6 should get further attention for future monitoring due to their surrounding anthropogenic activities to improve water quality as supported by earlier study (Rajonee,21 ).
Table 1: Average Value of Water Physico-Chemical Parameters in Six Stations of Kirtankhola River.
Parameter (Unit) | ST 1 | ST 2 | ST 3 | ST 4 | ST 5 | ST 6 |
SWT ( ÌŠC) | 18.1 | 17.7 | 18.6 | 18.0 | 18.6 | 17.7 |
pH | 8.15 | 8.10 | 7.75 | 8.05 | 7.80 | 8.45 |
DO (mg/l) | 5.22 | 6.55 | 7.40 | 6.65 | 4.20 | 4.80 |
EC (µS/cm) | 208 | 185 | 195 | 225 | 175 | 260 |
TDS (mg/l) | 220 | 185 | 195 | 325 | 185 | 355 |
NO3- (mg/l) | 0.95 | 0.80 | 0.85 | 0.75 | 0.60 | 1.15 |
NH4+ (mg/l) | 0.35 | 0.55 | 0.75 | 0.88 | 0.70 | 1.05 |
SO42- (mg/l) | 2.5 | 1.75 | 3.10 | 2.15 | 1.55 | 2.65 |
DIP (mg/l) | 3.25 | 2.75 | 3.78 | 3.70 | 3.65 | 4.10 |
CO3 2- (mg/l) | 15.22 | 16.90 | 22.25 | 17.70 | 21.12 | 14.15 |
Note: SWT Means Surface Water Temperature.
Phytoplankton Diversity
Species diversity is a reliable tool to assess the quality of an ecosystem based on relations between the number of taxa and the number of individuals. This research recorded a total of 53 phytoplankton species belongs to four classes, where Bacillariophyceae was dominant group following Euglenophyceae, Chlorophyceae and Cyanophyceae. The occurrence of Cyanophyeae was almost similar in all stations. ST 6 showed highest number of individuals (53), while minimum (20) was found in ST 5 (Table 2). Fragilaria brevistriata, Melosira granulata, Phacus caudatus, Pandorina morum and Scenedesmus acuminatus was found abundantly (cell/ml) in ST 1, 2, 4, 5 and 6. Scenedesmus acuminatus was found commonly in five stations except at ST 1, whereas Euglena chlamydophora, Gomphonema olivaceum and Cymbella parva found only in ST 1, 3 and 5. As single genus Euglena and Nitzschia possess highest number of taxa in this studied river. However, Margalef index indicated that ST 6 had highest species richness, while ST 4 had lowest (Figure 2 a). In case of species evenness by Simpson index, ST 2, 4 and 5 had few species that were dominant over the other species, but phytoplankton were evenly distributed in ST 1, 3 and 6 (Figure 2b). The Shannon Weiner index indicated the higher diversity in ST 3 and 6, while ST 4 and 5 had least diversity in species distribution (Figure 2 c). However, the three-diversity indices showed that diversity of phytoplankton in ST 6 was far better than the other stations.
Table 2: List of Phytoplankton and their Occurrence (Cell/ml) in the Studied Area.
Class | Taxa | ST 1 | ST 2 | ST 3 | ST 4 | ST 5 | ST 6 |
Cyanophyceae | Anabaena constricta (Szafer) Geit. | 2 | 1 | - | - | 1 | - |
Microcystis aeruginosa Kütz. | - | 1 | - | 1 | - | 1 | |
Merismopedia punctata Meyen | - | - | 1 | 1 | - | - | |
Oscillatoria limnetica Lemm. | 1 | - | 2 | - | 1 | - | |
Oscillatoria sancta (Kütz.) Gom. | - | 1 | - | - | - | 1 | |
Oscillatoria subbrevis Schmidle | 1 | - | - | 1 | 1 | 1 | |
Chlorophyceae | Actinastrum hantzchii Lagerh. | 1 | 1 | - | 2 | - | 1 |
Chlamydomonas gloeogama Kors. | 2 | 1 | 1 | - | - | 1 | |
Chlorella vulgaris Beyernick | - | - | 2 | 1 | - | - | |
Eudorina elegans Ehr. | - | - | - | - | 1 | 2 | |
Pandorina morum (Müll.) Bory | - | - | 3 | - | 2 | - | |
Planktosphaeria gelatinosa Smith | 1 | - | 1 | - | - | 3 | |
Scenedesmus acuminatus Chodat | - | 1 | 2 | 1 | 1 | 4 | |
Scenedesmus brasiliensis Bohlin | 1 | - | - | 2 | - | 1 | |
Scenedesmus quadricauda (Tur.) Bréb. | - | - | - | - | 1 | 1 | |
Scenedesmus regularis Svirenko | 2 | 2 | 1 | - | - | - | |
Euglenophyceae | Euglena acus (Müll.) Ehr. | 1 | - | 1 | - | - | - |
Euglena chlamydophora Mainx | 1 | - | - | - | - | 1 | |
Euglena clara Skuja | - | 1 | 2 | 1 | - | 1 | |
Euglena proxima Dangeard | - | - | - | - | 1 | 3 | |
Euglena variabilis Klebs | - | - | 1 | - | 1 | - | |
Euglenocapsa pisciformis Klebs | - | 2 | - | - | - | 1 | |
Phacus caudatus Hubn. | 2 | - | 1 | 3 | - | - | |
Phacus curvicauda Swirenko | 1 | 1 | - | - | 1 | 2 | |
Lepocinclis acus (Müll.) Mar. & Melk. | - | - | - | - | 1 | 1 | |
Trachelomonas armata (Ehr.) Stein | - | - | - | 1 | 1 | - | |
Trachelomonas hispida Lemm. | - | 1 | 1 | - | - | - | |
Trachelomonas oblonga Lemm. | 1 | 1 | - | - | - | 2 | |
Bacillariophyceae | Cymbella cistula (Ehr.) Kirch. | 2 | 1 | 2 | - | - | 1 |
Cymbella parva (W.Smith) Kirch. | - | - | - | - | 1 | - | |
Cyclotella bodanica Eulen. ex Grun. | - | - | - | - | 1 | 2 | |
Cyclotella comta (Ehr.) Kütz. | 1 | - | 2 | 1 | - | 1 | |
Fragilaria brevistriata Grun. | 3 | - | 1 | - | - | - | |
Fragilaria crotonensis Kitton | - | - | - | 1 | - | 1 | |
Fragilaria capucina Desm. | - | 1 | - | - | - | 1 | |
Fragilaria virescens Ralfs | - | 1 | 3 | - | - | 2 | |
Gomphonema fanensis Maillard | 1 | - | - | 2 | 1 | - | |
Gomphonema lanceolatum (Ehr.) Hust. | 1 | 1 | - | - | 1 | 2 | |
Gomphonema olivaceum (Horn.) Kütz. | - | - | 2 | - | - | - | |
Melosira distans (Ehr.) Kütz. | - | 1 | - | 2 | - | 2 | |
Melosira granulata (Ehr.) Ralfs | 1 | 3 | - | 2 | - | - | |
Melosira undulata (Ehr.) Kütz. | - | - | 2 | 1 | - | 1 | |
Navicula cuspidata Kütz. | 1 | 1 | - | - | - | 2 | |
Navicula decussis Oestrup | - | - | 1 | - | - | 3 | |
Navicula placentula (Ehr.) Grun. | 2 | - | - | 1 | 1 | 1 | |
Nitzschia acicularis W. Smith | - | 2 | 1 | - | - | - | |
Nitzschia clausii Hantz. | - | - | 2 | - | - | 2 | |
Nitzschia gracilis Hantz. | 1 | - | - | 1 | - | 1 | |
Nitzschia subrostrata Hustedt | - | 1 | 2 | - | 1 | - | |
Nitzschia subtubicola Germain | 2 | - | - | - | 1 | 1 | |
Surirella robusta Ehr. | - | - | 1 | 2 | - | 1 | |
Synedra tabulata (Ag.) Kütz. | 1 | - | 3 | - | - | - | |
Synedra ulna (Nitzch) Ehr. | - | - | 1 | 1 | - | 2 |
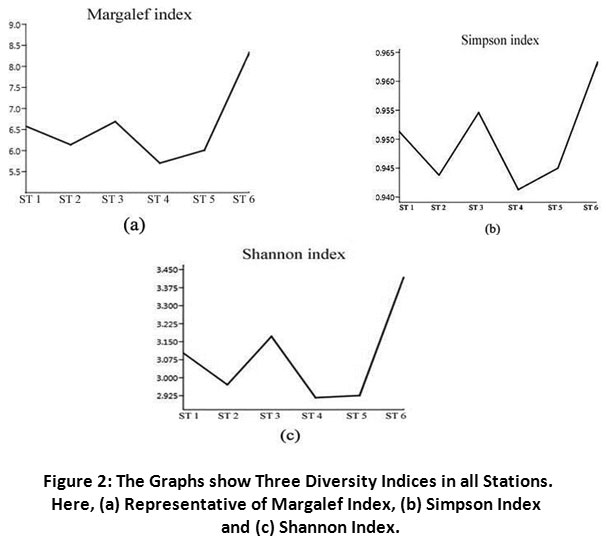 | Figure 2: The Graphs show Three Diversity Indices in all Stations. Here, (a) Representative of Margalef Index, (b) Simpson Index and (c) Shannon Index. Click here to view Figure |
Correlation Studies
The study attempted to make correlations among the measured physico-chemical parameters, also with the four groups of phytoplankton. There were significant and highly significant correlations among the measured parameters, and with the phytoplankton groups. However, there were no significant correlations in case of dissolve oxygen and dissolve inorganic phosphorus with other parameters except TDS. These two are important to measure impacts of water quality on aquatic biota. However, EC and TDS were significantly correlated with maximum parameters in physicochemical aspects in Kirtankhola River (Table 3). Increases of EC was linked with the increases of pH, TDS, NO3-, CO3 2- and NH4 +, and the decreases in temperature could increase the EC, TDS and pH values in the water. Moreover, NO3- negatively correlated with CO3 2- (r= -0.7128) but positively with SO42- (r= 0.6389). In case of phytoplankton group, the water quality had no impact on Cyanophyceae except NH4+ (r= -0.7247). That meant, presence of NH4+ is responsible for low occurrence of Cyanobacteria. On the other hand, EC, TDS, NO3- , NH4+ and DIP were responsible for presence of Chlorophyeae. Temperature and CO32- was sensitive for the occurrence of Euglenophyeae, although increasing pH, EC, TDS, NO3-, and SO42- favored their abundance, while Bacillariophyeae positively correlated with EC, TDS, NO3-, NH4+ and DIP.
Table 3: Correlation Coefficient Between Physico-Chemical Parameters and Different Phytoplankton Groups at 5% Probability Level.
| Temp (ºC) | pH | DO (mg/l) | EC (mS/cm) | TDS (mg/l) | NO3- (mg/l) | NH4+ (mg/l) | SO42- (mg/l) | DIP (mg/l) | CO32- (mg/l) | |
| Temp | 1 | |||||||||
| pH | -0.8765 | 1 | ||||||||
| DO | 0.0058 | -0.3154 | 1 | |||||||
| EC | -0.5767 | 0.8059 | -0.1184 | 1 | ||||||
| TDS | -0.5366 | 0.7075 | -0.1195 | 0.9446 | 1 | |||||
| NO3- | -0.5766 | 0.8001 | -0.0629 | 0.7987 | 0.5630 | 1 | ||||
| NH4 + | -0.1487 | 0.2638 | -0.0174 | 0.6337 | 0.7319 | 0.2527 | 1 | |||
| SO42- | 0.1062 | 0.1023 | 0.4096 | 0.4708 | 0.2653 | 0.6389 | 0.2085 | 1 | ||
| DIP | 0.2611 | 0.0931 | -0.2176 | 0.3918 | 0.6155 | 0.2879 | 0.3874 | 0.4779 | 1 | |
| CO3 2- | 0.3730 | -0.9589 | 0.3099 | 0.6809 | 0.3841 | -0.7128 | -0.0107 | 0.0172 | 0.1098 | 1 |
| CYN | -0.0201 | 0.1921 | -0.2297 | 0.0000 | 0.1559 | 0.2579 | -0.7247 | 0.1800 | -0.2988 | -0.4073 |
| CHL | -0.1636 | 0.4649 | -0.0182 | 0.7426 | 0.5535 | 0.8266 | 0.5887 | 0.3830 | 0.6980 | -0.2653 |
| EUG | -0.6058 | 0.8066 | -0.1302 | 0.8141 | 0.6205 | 0.9434 | 0.4813 | 0.5133 | 0.3858 | -0.6477 |
| BAC | -0.2195 | 0.4534 | 0.1879 | 0.7324 | 0.5288 | 0.8539 | 0.5038 | 0.3717 | 0.5852 | -0.2821 |
CYN= Cyanophyceae, CHL= Chlorophyceae, EUG= Euglenophyceae, BAC= Bacillariophyceae.
Note: Here, Correlations among the Phytoplankton Groups were Discarded.
Conclusion
This study indicates that the water of Kirtankhola River is in good quality, although some locations (ST 3 and 6) are being contaminated due to discharges from industries, water vehicles and other anthropogenic activities. In total 53 phytoplankton taxa were recorded under four classes and Bacillariophyceae is dominant over the other groups and the water of ST 6 showed good biodiversity indices comparatively than others. However, this is the first report on phytoplankton diversity and correlation among water physico-chemical parameters with phytoplankton groups in this river. The water quality has mostly positive impacts on Chlorophyceae and Bacillariophyceae.
Acknowledgement
The authors are grateful to the supports provided by Muhammad Risalat Rafiq, Assitant Professor at the Department of Geology and Mining, University of Barishal. And also thankful to Shawon Mitra, Lecturer, Department of Botany, University of Barishal.
Funding Source
The research was conducted without financial supports and all costs were provided by the authors.
Conflict of Interest
Authors did not have any conflict of interest.
References
- Mahbub M M. Kirtankhola River. Banglapedia, The National Encyclopedia of Bangladesh. 2014. http://en.banglapedia.org/index.php?title=Kirtankhola_River
- Kumar A and Dua A. Water Quality Index for assessment of water quality of River Ravi at Madhopur, India. Global Journal of Environmental Sciences. 2009. 8(1):49-57.
CrossRef - Partensky F, Blanchot J, Vaulot D. Differential distribution and ecology of Prochlorococcus and Synechococcus in oceanic waters: A review. Bulletin Institute of Oceanography. 1999. 19:457-475.
- Reynolds C, Huszar V, Kruk C, Naselli-Flores L, and Melo S. Towards a functional classification of the freshwater phytoplankton. J. Plankton Res. 2002. 24:417-428.
CrossRef - Brettum P and Andersen T. The use of phytoplankton as indicators of water quality. NIVA report SNO 2005. 4818-2004.
- Maguran A E. Ecological diversity and its measurement. Chicago University Press, Chicago, USA. 1983. 111.
- Purvis A and Hector A. Getting the measure of biodiversity. Nature. 2000. 405:212–219.
CrossRef - Shannon C E and Weiner W. The mathematical theory of communication. Urbana, IL. University of Illinois Press. 1949.
- Spellerberg, Ian F and Peter J. A tribute to Claude Shannon (1916–2001) and a plea for more rigorous use of species richness, species diversity and the ‘Shannon–Wiener’Index. Global ecology and biogeography. 2003. 12(3):177-179.
CrossRef - Simpson E H. Measurement of diversity. Nature. 1949. 163:688.
CrossRef - Newall E J L, Chu V T, Pringault O, Amouroux D, Arfi R, Bettarel1 Y, Bouvier T, Bouvier C, Got P, Nguyen T M H, Mari X, Navarro P, Duong T N, Cao T, Pham T, Ouillon S, Torreton P. Phytoplankton diversity and productivity in a highly turbid, tropical coastal system(Bach Dang Estuary, Vietnam). Biogeosciences Discussion. 2011. 8:487–525.
CrossRef - Vallina S M, Follows M J, Dutkiewicz S, Montoya J M, Cermeno P, Loreau M. Global relationship between phytoplankton diversity and productivity in the ocean. Natural Community. 2014. 5:4299.
CrossRef - Whitton B A and Patts M. The Ecology of Cyanobacteria. Kluwer Academic. Dordrecht. 2000.
- Akbay N, Anul, Yerti, Soyupak and Yurteri. Seasonal distribution of large phytoplankton in Keban Dam Reservoir. Plankton Research. 1999. 21(4):771-787.
CrossRef - Peerapornpisal Y, Sonthichai W, Somdee T, Mulsin P and Rott E. Water Quality and Phytoplanktonin the Mae Kuang Udomtara Reservoir, Chiang Mai, Thailand. Journal of Science Faculty of Chiang. 1999. 26(1):25-43.
CrossRef - Elliott J A, Irish A E and Reynolds C S. Predicting the spatial dominance of phytoplankton in light limited and incompletely mixed eutrophic water column using the Protech Model. Freshwater Biology. 2002. 47(3):433-440.
CrossRef - Goldman C R, Horne A J. Limnology. McGraw-Hill Book Company. New York, USA. 1983. 464.
- Islam A K M N and Alfasane M A. Euglenophyceae from Barisal district, Bangladesh: III. Genus Trachelomonas Ehr. Bangladesh Journal of Plant Taxonomy. 2004. 11:33-37.
- Khondker M, Bhuiyan R, Yeasmin, J, et al. New records of phytoplankton for Bangladesh. 2. Cryptophyceae and Synurophyceae. Bangladesh J. Bot. 2006. 35:53-59.
CrossRef - Chakraborty S, Karmaker D, Das SK and Hossen R. First report on phytoplankton communities of Barishal City, Bangladesh. Current Botany. 2020. 11:142-147.
CrossRef - Rajonee A A. Surface water quality status around Barisal city in two different seasons. Bangladesh J. Soil Sci. 2018. 40(2):1-14.
- APHA (American Public Health Association). Standard methods for the examination of water and wastewater (19th eds.). Washington DC. USA. 1998.
- Bellinger E G and Sigee D C. Freshwater algae: identification and use as bioindicators. John Wiley & Sons, USA. 2010. 244.
CrossRef - Ahmed Z U, Khondker M, Begum ZNT, et al. Encyclopedia of flora and fauna of Bangladesh. Asiatic Soc. Bangladesh, Dhaka. 2009. 543.
- Islam A K M N and Moniruzzaman K. Contribution to the study on Euglenophyta of Bangladesh. I. Genus Trachelomonas Ehr. Internationale Revue der gesamten Hydrobiologie. 1981. 66:109-125.
CrossRef - Smith G M. Freshwater algae of the United States, New York. 1950. 719.
- Komárek J and Fott B. Chlorococcales (Huber-Pestalozzi, Eds.), Das Phytoplankton des Süsswassers, Systematik u. Biologie, Teil 1, Stuttgart. 1983. 1044.
- Bold H C and Wynne M J. Introduction to the Algae, Prentice-Hall, New Jersey. 1985. 706.
- WHO (World Health Organization). Guidelines for drinking water quality (electronic resource, 3rd Eds.). 2008. 1(53):30-113.






