Existence of Microplastic as Pollutant in Harike Wetland: An Analysis of Plastic Composition and First Report on Ramsar Wetland of India
DOI: http://dx.doi.org/10.12944/CWE.16.1.12
Copy the following to cite this article:
Manzoor S, Kaur H, Singh R. Existence of Microplastic as Pollutant in Harike Wetland: An Analysis of Plastic Composition and First Report on Ramsar Wetland of India. Curr World Environ 2021;16(1). DOI:http://dx.doi.org/10.12944/CWE.16.1.12
Copy the following to cite this URL:
Manzoor S, Kaur H, Singh R. Existence of Microplastic as Pollutant in Harike Wetland: An Analysis of Plastic Composition and First Report on Ramsar Wetland of India. Curr World Environ 2021;16(1). Available From : https://bit.ly/3sCt9TI
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 21-06-2020 |
|---|---|
| Accepted: | 02-03-2021 |
| Reviewed by: | 
 Norlaila Mohd Zanuri
Norlaila Mohd Zanuri
|
| Second Review by: |

 Kingshuk Dutta
Kingshuk Dutta
|
| Final Approval by: | Dr. Hiren B. Soni |
Introduction
Wetlands are having many global processes such as carbon cycle, fisheries production, shelter for local and migratory birds and biogeochemical cycles. They are not only ecologically important but also contribute in economy of that area. Wetland export 20% of total world’s organic carbon in nature. It also contributes to biomass production where in animals although small but form an important fraction of this biomass. Wetlands particularly riverine wetlands provide important nursery area and food sources for aquatic fauna. All wetlands have same common nature where water levels fluctuate up and down, desalination site of salty water, high primary and secondary productivity, high export of materials to other ecosystem, nurseries and shelter of birds. In addition, it also acts as refuses and corridors for terrestrial animals, gene banks for many plants and animal species along with economic services for human beings. Society also considers wetlands for their historical legacy in sediments of climate, recreation, relaxation and location for solid waste disposal. So, wetlands are more important in global process than their aerial extent calculation1.
In India, there are 67,429 wetlands, covering about 4.1 million hectares. Out of which, 2,175 are natural wetlands, covering an area of 1.5 million hectares, and rest 65,254 are man-made, wetlands covering 2.6 million hectares of area 2. Out of the 65,254 man- made wetlands, Harike wetland is one of the largest wetlands of northern India which is at the confluence of 3 districts: Amritsar, Ferozpur and Kapurthala. This wetland came into existence in 1952 due to diversification of water resources of two rivers; Sutlej and Beas. It was declared as a Ramsar site by International Union for Conservation of Nature (IUCN) in 1990 giving it a shape of wetland of international significance and priority zone for biodiversity conservation. Furthermore, it was declared as bird sanctuary in 1992 3. This wetland is sourced by two rivers namely Sutlej and Beas which helps in rejuvenating ground water along with providing irrigation. The area of wetland was 148km2 which is restricted to 86km2 at present, out of which 45km2 is dry area and 41km2 wet zone. The wet territory also stands diminished to 28km2 with real water spread inside wet zone is 17-18km2 and rest is marshy. 44.4% of the wet zone is covered by water hyacinth. Wetland degradation was attributed to siltation, water hyacinth invasion and pollution brought in mainly by river Sutlej and less by river Beas. These threats not only result in area decreasing of wetland but also decrease quality of natural environment as required for survival of fauna4.
Overuse of nature is common tendency of human beings for self-benefit. Now direct exploitation is controlled and monitored by local and national regulatory bodies, but indirect impact of our routine life needs to be analyzed. By routine life, society is likely to generate new stressor for environment. Microplastic is one of the emerging stressors generated by our routine life. They are plastic fibers and particles are formed after the degradation or fragmentation of synthetic polymers with a size range of <5mm5. They were first reported in marine surface water in 1970s 6, 7. Since microplastics have been found globally8, accumulation of microplastics in freshwater is also getting in focus for research 9, 10, 11, and 12. Presence of microplastic in the ecosystem brings various consequences such as oral uptake, damaging tissue, surface for attachment and transport of contaminants 13, 14. The chemistry of microplastic may get modified by leaching out of additives from it and by the adsorption of various toxic substances from water onto its surface due to hydrophobic attraction 15. Due to large surface to volume ratio, they accumulate persistent bioaccumulative toxic compounds, heavy metals and so on converting into a multiple stressor16. These microplastic particles with adsorbed contaminants are ingested by a number of organisms17, 18, 19, and 20. Microplastic mimics the algal derived compounds such as dimethyl sulphide which is a threat for organism which feeds on the algae using chemosensory mechanism 21. It has been also found that microplastics have the capacity to block digestive tract, inducing toxicity and translocating from gut to circulatory system in Mytilus Edulis (L.)22, 23. The presence of microplastic in animals is of concern because consumption of contaminated fish or food may act as a vector for translocation of microplastics in humans thereby contaminating foodweb24. Microplastics have also been found to effect and alter homeostasis in the body of fish as studied by Rainieri 25 who supplemented Zebra fish feed with microplastics along with a set of contaminants.
Approximately 5.6 million tonnes per annum plastic waste is generated in India by different sectors including products and packaging. Packaging represents the single largest sector of plastic use and accounts for 35% of plastic consumption and greatly increased plastic waste and consequently litter. Out of total production 80% plastics are recyclable and remaining 20% are nonrecyclable plastic. Large aquatic bodies are recognized as hot spot for microplastic pollution. There is no study yet that have reported the presence of microplastics in any wetland of India. Current study is an attempt to confirm presence of microplastic in largest wetland of northern India being ecological and economical important.
Methodology
Reference Data for Plastic Composition Identification
To prepare the reference database for the need of comparing the sample results from established techniques, different type of virgin plastic beads from Hi-Tech polymers limited was brought and analyzed through the said techniques. Spectra obtained from each technique were kept as a reference spectrum to compare with sample results.
Study Area
Harike Wetland is manmade, riverine and lacustrine wetland located between latitudes 31°05’15” and 31 14’15” N and longitudes of 74°55’ 30” and 75°07’30” E. This wetland is drained by Sutlej and Beas rivers along with their tributaries. It holds importance in recharging ground water long with acting as a source of irrigation to parts of Punjab and Rajasthan through Sirhind feeder and Rajasthan canal. In the months of April, May and June, it encounters the extreme heat with maximum daily temperature rising upto 43°C in June followed by daily min temperature as 0.6°C in January26. Khaitan area is a core area of harike wetland so it was marked as sampling site in current work.
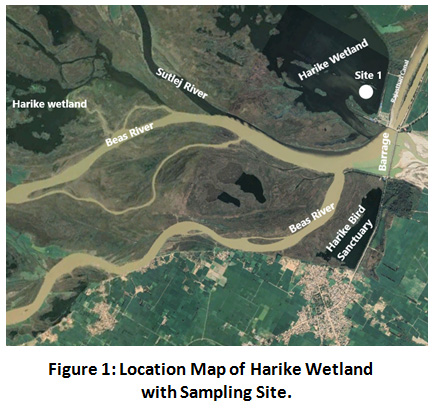 |
Figure 1: Location Map of Harike Wetland with Sampling Site. Click here to view Figure |
Sampling and Recovery of Microplastic
Surface water sampling was conducted from Khaitan area (Site 1, marked area) in the month of May 2018 by fixing plankton net to local boat, which assured a particular position of net towards the water surface. The net of mesh size 50µm was dragged to an area of 600m which is representing ecological condition of wetland up to the depth of 15cm. After sampling the net was carefully packed to prevent secondary contamination and transferred to laboratory for further work.
In the laboratory net was washed with distilled water and further steps were carried out in laminar and in glassware to prevent any contamination. Organic and synthetic macroparticles were visually sorted out of which plastic was confirmed by hot needle test 27. Following the separation of macroparticles washoff was passed through different mesh size filter (305, 200, 100, 60µm) to separate particle on basis of their size range. After that each residue was treated with 30% hydrogen peroxide for seven days to digest organic matter28 and at the last of recovery step, sodium iodide (density 1600mg/L) was used for density-based separation29. The samples were kept in oven at 55 ºC for 20min to remove moisture and equally divided for further analysis. For the identification of polymer type by vibrational spectroscopy 30 data has been followed.
Fourier Transform Infrared Spectroscopy (FTIR)
FTIR is most widely used technique due to its versatility in giving information regarding composition and confirmation of plastic 31. FTIR measurement of the isolated particles and sample residues were measured by 8400S-Shimadzu FTIR Spectrometer equipped with a software IR solution. The spectra of every particle and sample residues were recorded in a wave number range of 4000-500cm-1 in transmittance mode with a spectral resolution of 4cm-1 coadded with 15 scans.
Raman Spectroscopy
Raman Spectroscopy records the characteristic peaks in response to polymer type in sample. It is based on a monochromatic light (laser source) interacting with the sample. Raman analysis of the sample residues were performed by a STR500 confocal Raman Spectrometer. 1mg of each sample was analyzed through a fixed wavelength of 532nm. Spectra of samples were recorded in the wave number range of 100-5000cm-1, with power of 2.5, 5 and 12.5mW. Four scans were accumulated for each spectrum.
Gas Chromatography - Mass Spectrometry
GC-MS confirms the polymer type by means of characteristic decomposition products. For GC-MS, the mass fragmentation pattern of compounds in samples was compared with the main fragmentation pattern of virgin plastics 32. As the degradation pattern of polymer remains fixed 32, identification of compounds was carried out by studying and comparison of mass spectra of sample with standard indicator ion peaks33. The analysis was performed on NUCON 5700 Gas Chromatograph with a Mass Spectrometer. The instrument was fitted with a programmable injector the temperature of which was set at 280°C. GC column with dimensions 30 m x 0.25 mm x 0.25 mm was temperature programmed from 100°C to 260°C at 8°C/min, then to 340°C at 35°C/min maintained for 23 min. The carrier gas, Helium was set to be injected as 0.001L with an interval of 60 seconds at the injector port. 34 protocol has been followed for the sample analysis.
Results and Discussion
During lab work total 15 particles were manually separated at initial stage, out of which two particles were discarded due to their large size >12mm.
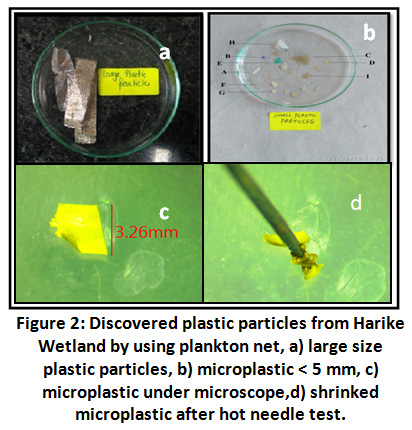 |
Figure 2: Discovered Plastic Particles from Harike Wetland by using Plankton Net. Click here to view Figure |
Remaining thirteen particles were subjected to hot needle test and out of them nine were confirmed as plastic and rest as non- plastic organic matter. The test was such that a hot needle coming in contact with the particle made it either to melt or shrink thereby confirming it as plastic 27. These confirmed nine plastic particles were given the alphabetic code as particle A, B, C, D, E, F, G H, and I for future correspondence.
FTIR Characterization
FTIR spectrum of these nine particles (A-I) were matched with spectrum of virgin plastic beads. Spectra of particles except C were comparable with standard amide (Nylon 6) spectrum. Bands at 1500cm-1, 1600cm-1 and 3400cm-1 , NH bending and C=O stretching for amide I and amide II were taken as reference peak for nylon 6. Nylon 6 is one of the most common polyamides used mostly in household and industrial products. The Infrared spectra of nylon 6 features 3300cm-1 for NH stretch band accompanied by a weak peak at around 3050cm-1 and assigned to fermi split pair of NH stretch. On other hand two other bands were found at 1630cm-1 and 1550cm-1corresponding to amide-I and amide-II band respectively. The naming convention for Polyamides relies on number of carbon atoms an acid and amino component holds to form the amide. Nylon 6 IS usually formed by two approaches: Dicarboxylic acid and a diamine 30. With the help of IR, identification of Nylon 6 samples corresponding to a Polyamide is important but can be difficult due to the polymorphism exhibited by some Polyamides.
Whereas in particle C, peaks at 2925cm-1 (asymmetric CH2 stretch) and 2854cm-1 (symmetrical, CH2 stretch) were observed which is indication of high-density polyethylene (HDPE) chain usually methyl group and bending modes of group CH2 observed at 1462cm-1. Addition to this two characteristic peaks of polyethylene HDPE between 1300-1400cm-1were considered i.e. 1347cm-1 and 1381cm-1 and no individual peak at 1377cm-1 was observed which is characteristics of LDPE. For confirmation spectra of Particle C were merged with Virgin HDPE. By this way generated data through FTIR confirmed eight particles as Nylon 6 and one as Polyethylene.
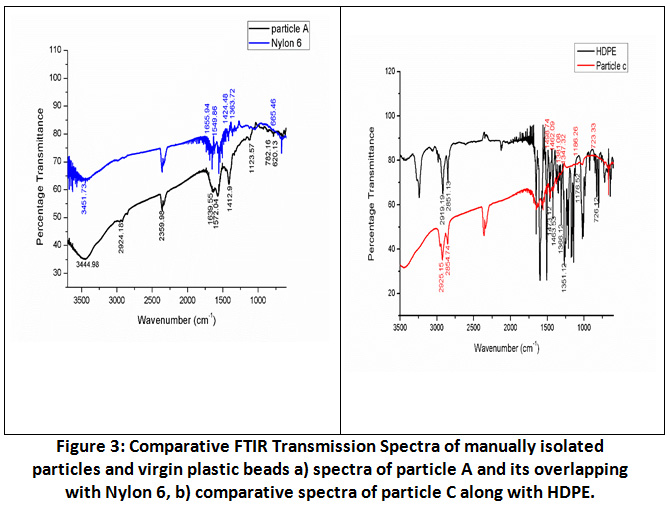 |
Figure 3: Comparative FTIR Transmission Spectra of Manually Isolated Particles and Virgin Plastic Beads. Click here to view Figure |
Sample residues obtained from filtration through varying mesh size filter cloth after organic digestion and density-based separation, were also subjected to FTIR analysis. The spectra of samples were overlapped with that of plastic particle A and C, out of which the particle A FTIR spectra was matching with that of all i.e. 305, 200, 100, 60μm residue in terms of characteristic Nylon 6 Peaks. Generated merged spectra were also compared parallel to virgin Nylon 6 spectrum to which all four merged together gave a clear picture about the presence of characteristic Amide groups in sample.
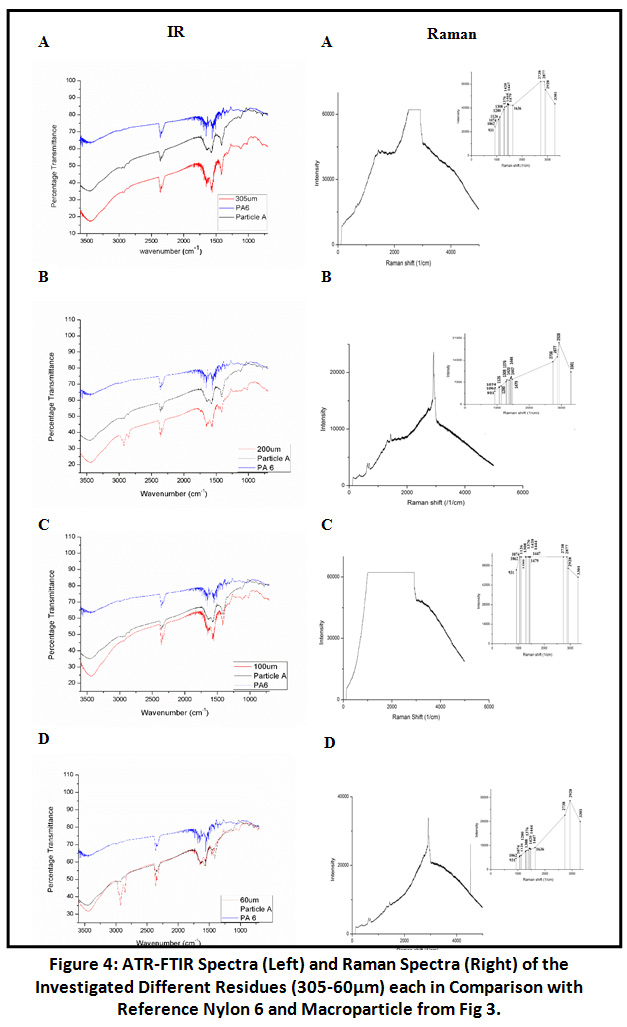 |
Figure 4: ATR-FTIR Spectra (Left) and Raman Spectra (Right) of the Investigated Different Residues. Click here to view Figure |
The spectra of all residues were also compared with particle C which was earlier identified as polyethylene as well as parallel comparison with virgin Polyethylene spectrum because probability for the presence of nylon 6 and HDPE in residues was more as they were earlier identified at visual stage (Particle A-I).
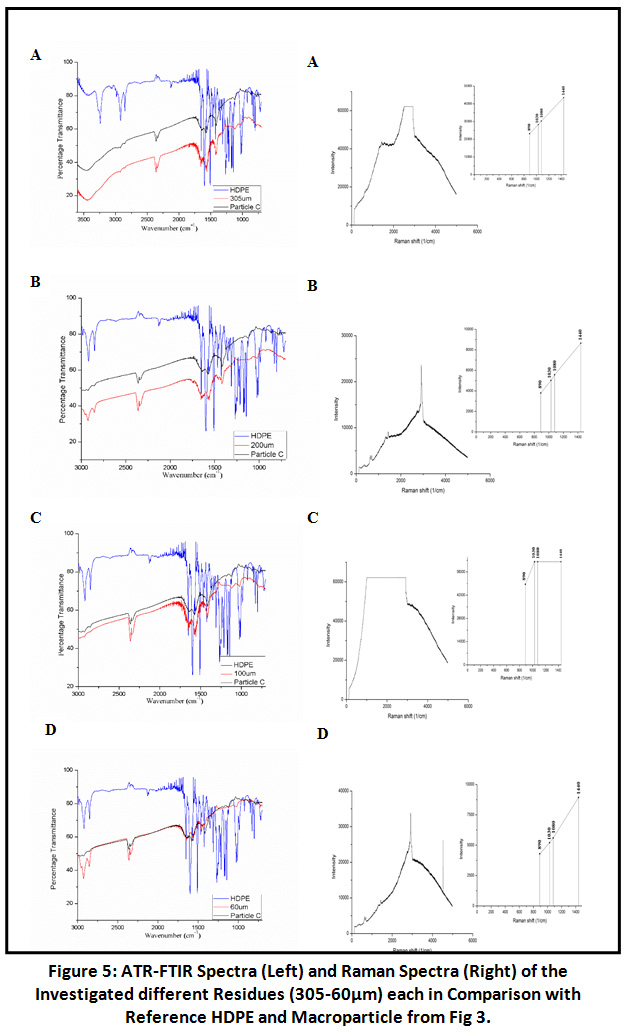 |
Figure 5: ATR-FTIR Spectra (Left) and Raman Spectra (Right) of the Investigated Different Residues. Click here to view Figure |
Spectra of 305µm and 60µm particle size residues were indicating for polyethylene due to the presence of the typical two polyethylene (HDPE) peaks at 2925cm-1 (asymmetric CH2 stretch) and 2854cm-1 (symmetrical, CH2 stretch), 1462cm-1 (CH2 bending) and two characteristic peak of polyethylene HDPE between 1300- 1400cm-1. For further confirmation for the presence of Nylon 6 and Polyethylene sample examined through RAMAN Spectroscopy and GC-MS.
Raman Spectroscopic Analysis
Results of FTIR were further validated by RAMAN Spectroscopy. Spectra at 931cm-1 and 3301cm-1 were observed in screened sample residues (305, 200, 100, 60µm) for C-CO stretch and NH stretch as characteristics of Nylon 6. 1126cm-1 for C-C stretch and other characteristic peaks were also observed [Fig 4]. In addition to the Nylon 6 there was also the indication of polyethylene in the sample residues (305, 200, 100, 60µm) because of the presence of spectrum include 890cm-1 for C-H stretch and peaks at 1030cm -1 and 1080cm -1 due to C- C stretch vibrations. Another additional peak is at 1440cm -1 which is due to CH2 bending [Fig 5]. The results obtained were compared with the RAMAN spectra of virgin Nylon 6 and HDPE for proper validation of sample residues spectra. Hence, we can conclude that the RAMAN further supported the results given by FTIR about the presence of polyethylene.
Gas Chromatography and Mass Spectrometry
For GC-MS only two sample residues, 305 and 60µm were further considered. The study was only carried out for 305µm and 60µm as they set a size range.
Nylon 6 polymer degrades in number of compounds and gives mass spectrum with the components of different carbon numbers such as 1-pentenenitrile, €-caprolactam, and N-5-cyanopentyl-6- hexanamide fragments. Presence of €-caprolactam was found by its indicator peaks m/z 30, 42, 55, 67, 85, 98, and 113 32. Hence by the presence of indicator ion peak in degraded form 305µm and 60µm confirmed presence of Nylon 6 in the sample in microform 37.
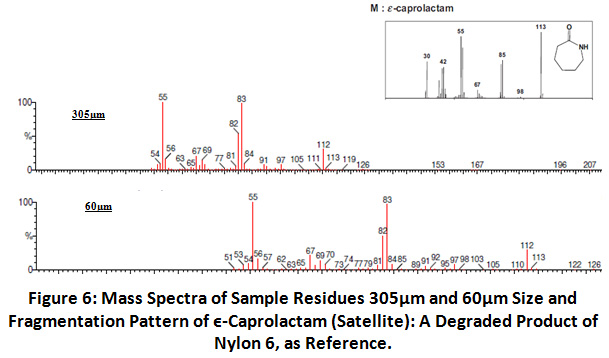 |
Figure 6: Mass Spectra of Sample Residues 305µm and 60µm Size and Fragmentation Pattern of ϵ-Caprolactam (Satellite): A Degraded Product of Nylon 6, as Reference. Click here to view Figure |
In addition, the results also indicated the presence of polyethylene (HDPE). Literature shows that the degradation mass fragments of polyethylene polymer includes 1-heptene, 1, 9-decadiene, 1-eicosene 32. The indicator ion peaks for C20: 1-eicosene in sample 305µm and 60µm are obtained at m/z 29, 41, 55, 69, 83, 97, and 111.
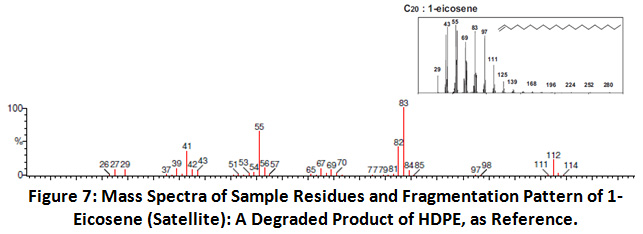 |
Figure 7: Mass Spectra of Sample Residues and Fragmentation Pattern of 1-Eicosene (Satellite): A Degraded Product of HDPE, as Reference. Click here to view Figure |
Hence as the indicator ion peaks for the presence of C20: 1-eicosene was found in the samples therefore also indicating the presence of polyethylene in the sample residues 37. These results confirm that Harike wetland is also contaminated by anthropogenic litter. Source of contamination might be lateral inputs from land which could be occurred through washout of rain or by air. Wetland is conserved area so no direct human impact can elevate level of contaminants but both rivers who are receiving sewage inputs during their course can be responsible for this. Use of nylon 6 bags are common practice on dike of inland water bodies in India, these bags also degrade and become source of microplastic in wetland. In bank deposition of organic contents stuck plastic waste where it can fragment, degrade and convert in microsize. Some plastic products because of high density sink and settle in bottom but after degradation redistribute on surface. Nylon 6 and HDPE are detected in wetland here which is indicating their dominancy over other plastics.
Conclusion
This study aimed to analyze the presence and composition of microplastic in Harike wetland. In addition, the polymer type was found to be nylon 6 and HDPE. This may reveal the various possible sources along and across the wetland resulting in microplastic pollution. The presence of microplastic is clearly indicate for the ingestion of these particles by the diverse organisms in the wetland. Representing a threat to biodiversity, the effect of microplastic on aquatic organisms must be considered. Further microplastic can adsorb harmful contaminants from the surroundings, therefore acting as a multiple stressor. Microplastic along with other contaminants may affect the organism indirectly which may further spread along the foodchain. Further investigation needs to incorporate the capability of microplastic to adsorb surrounding contaminants and their effects on the organisms. The hazardous effects of microplastic must be summarized to emphasize on further studies to reduce the effect of microplastic pollution effectively. This study is helpful to connect the knowledge gaps for proper understanding of the microplastic pollution in inland freshwater environments.
Conflict of Interest
The authors declare that they have no conflict of interest.
Aknowledgement
Authors would like to thank to technical staff of Harike wetland for their support during sampling of water.
References
- Andrady AL. Microplastics in the marine environment. Marine Pollution Bulletin. 2011;62(8):1596-1605.
CrossRef - Armentano TV. Drainage of Organic Soils as a Factor in the World Carbon Cycle. BioScience. 1980;30(12):825-830.
CrossRef - Besseling, E., Wegner, A., Foekema, E. M., Van Den Heuvel-Greve, M. J., & Koelmans, A. A. Effects of microplastic on fitness and PCB bioaccumulation by the lugworm Arenicola marina (L.). Environmental science & technology. 2013; 47(1): 593-600.
CrossRef - Browne, M. A., Crump, P., Niven, S. J., Teuten, E., Tonkin, A., Galloway, T., & Thompson, R. Accumulation of microplastic on shorelines woldwide: sources and sinks. Environmental science & technology. 2011; 45(21): 9175-9179.
CrossRef - Browne, M. A., Dissanayake, A., Galloway, T. S., Lowe, D. M., & Thompson, R. C. Ingested microscopic plastic translocates to the circulatory system of the mussel, Mytilus edulis (L.). Environmental science & technology. 2008; 42(13): 5026-5031.
CrossRef - Carpenter, E. J., Anderson, S. J., Harvey, G. R., Miklas, H. P., & Peck, B. B. Polystyrene spherules in coastal waters. Science. 1972; 178(4062): 749-750.
CrossRef - Carpenter, E. J., & Smith, K. L. Plastics on the Sargasso Sea surface. Science. 1972; 175(4027): 1240-1241.
CrossRef - Chopra, R., Verma, V. K., & Sharma, P. K. Mapping, monitoring and conservation of Harike wetland ecosystem, Punjab, India, through remote sensing. International Journal of Remote Sensing. 2001; 22(1): 89-98.
CrossRef - Claessens, M., Van Cauwenberghe, L., Vandegehuchte, M. B., & Janssen, C. R. New techniques for the detection of microplastics in sediments and field collected organisms. Marine pollution bulletin. 2013; 70(1-2): 227-233.
CrossRef - Cole, M., Lindeque, P., Fileman, E., Halsband, C., Goodhead, R., Moger, J., & Galloway, T. S. Microplastic ingestion by zooplankton. Environmental science & technology. 2013; 47(12): 6646-6655.
CrossRef - De Witte, B., Devriese, L., Bekaert, K., Hoffman, S., Vandermeersch, G., Cooreman, K., & Robbens, J. Quality assessment of the blue mussel (Mytilus edulis): Comparison between commercial and wild types. Marine pollution bulletin. 2014; 85(1): 146-155.
CrossRef - Della Torre, C., Bergami, E., Salvati, A., Faleri, C., Cirino, P., Dawson, K. A., & Corsi, I. Accumulation and embryotoxicity of polystyrene nanoparticles at early stage of development of sea urchin embryos Paracentrotus lividus. Environmental science & technology. 2014; 48(20): 12302-12311.
CrossRef - Do Sul, J. A. I., & Costa, M. F. The present and future of microplastic pollution in the marine environment. Environmental pollution. 2014; 185: 352-364.
CrossRef - Duncan, E. M., Broderick, A. C., Fuller, W. J., Galloway, T. S., Godfrey, M. H., Hamann, M., ... & Santillo, D. Microplastic ingestion ubiquitous in marine turtles. Global change biology. 2019; 25(2): 744-752.
CrossRef - Fischer, M., & Scholz-Böttcher, B. M. Simultaneous trace identification and quantification of common types of microplastics in environmental samples by pyrolysis-gas chromatography–mass spectrometry. Environmental science & technology. 2017; 51(9): 5052-5060.
CrossRef - Free, C. M., Jensen, O. P., Mason, S. A., Eriksen, M., Williamson, N. J., & Boldgiv, B. High-levels of microplastic pollution in a large, remote, mountain lake. Marine pollution bulletin. 2014; 85(1): 156-163.
CrossRef - Gulmine, J. V., Janissek, P. R., Heise, H. M., & Akcelrud, L. Polyethylene characterization by FTIR. Polymer testing. 2002; 21(5): 557-563.
CrossRef - Lechner, A., Keckeis, H., Lumesberger-Loisl, F., Zens, B., Krusch, R., Tritthart, M., ... & Schludermann, E. The Danube so colourful: a potpourri of plastic litter outnumbers fish larvae in Europe's second largest river. Environmental pollution. 2014; 188: 177-181.
CrossRef - Lobo, H., & Bonilla, J. V. (Eds.). Handbook of plastics analysis. 2003; (Vol. 68). Crc Press.
CrossRef - Mattsson, K., Ekvall, M. T., Hansson, L. A., Linse, S., Malmendal, A., & Cedervall, T. Altered behavior, physiology, and metabolism in fish exposed to polystyrene nanoparticles. Environmental science & technology. 2015; 49(1): 553-561.
CrossRef - Moza, U., & Mishra, D. N. Current status of Harike wetland vis a vis its ecology and fishery. Sengupta M and Dalwani R. eds. Proceedings of Taal. 2007; 1470-1476.
- Nuelle, M. T., Dekiff, J. H., Remy, D., & Fries, E. A new analytical approach for monitoring microplastics in marine sediments. Environmental Pollution. 2014; 184: 161-169.
CrossRef - Ogata, Y., Takada, H., Mizukawa, K., Hirai, H., Iwasa, S., Endo, S., ... & Murakami, M. International pellet watch: global monitoring of persistent organic pollutants (POPs) in coastal waters. 1. Initial phase data on PCBs, DDTs, and HCHs. Marine pollution bulletin. 2009; 58(10): 1437-1446.
CrossRef - Procter, J., Hopkins, F. E., Fileman, E. S., & Lindeque, P. K. Smells good enough to eat: Dimethyl sulfide (DMS) enhances copepod ingestion of microplastics. Marine pollution bulletin. 2019; 138: 1-6.
CrossRef - Rainieri, S., Conlledo, N., Larsen, B. K., Granby, K., & Barranco, A. Combined effects of microplastics and chemical contaminants on the organ toxicity of zebrafish (Danio rerio). Environmental research. 2018; 162: 135-143.
CrossRef - Ramachandra, T. V. Restoration and management strategies of wetlands in developing countries. 2001.
CrossRef - Setälä, O., Fleming-Lehtinen, V., & Lehtiniemi, M. Ingestion and transfer of microplastics in the planktonic food web. Environmental pollution. 2014; 185: 77-83.
CrossRef - Tanaka, K., Takada, H., Yamashita, R., Mizukawa, K., Fukuwaka, M. A., & Watanuki, Y. Accumulation of plastic-derived chemicals in tissues of seabirds ingesting marine plastics. Marine pollution bulletin. 2013; 69(1-2): 219-222.
CrossRef - Tsuge, S., Ohtani, H., & Watanabe, C. Pyrolysis-GC/MS data book of synthetic polymers: pyrograms, thermograms and MS of pyrolyzates. 2011; Elsevier.
- Watts, A. J., Lewis, C., Goodhead, R. M., Beckett, S. J., Moger, J., Tyler, C. R., & Galloway, T. S. Uptake and retention of microplastics by the shore crab Carcinus maenas. Environmental science & technology. 2014; 48(15): 8823-8830.
CrossRef - Watts, A. J., Urbina, M. A., Goodhead, R., Moger, J., Lewis, C., & Galloway, T. S. Effect of microplastic on the gills of the shore crab Carcinus maenas. Environmental science & technology. 2016; 50(10): 5364-5369.
CrossRef - Zbyszewski, M., & Corcoran, P. L. Distribution and degradation of fresh water plastic particles along the beaches of Lake Huron, Canada. Water, Air, & Soil Pollution. 2011; 220(1-4): 365-372.
CrossRef






