Concentration Dependent Effects of Green Inhibitors on Gravimetric Indices of Corrosion Linked Metal Integrity
DOI: http://dx.doi.org/10.12944/CWE.16.1.13
Copy the following to cite this article:
Olowoyo D. N, Fadairo E. A, Aziza, A. E. Concentration Dependent Effects of Green Inhibitors on Gravimetric Indices of Corrosion Linked Metal Integrity. Curr World Environ 2021;16(1). DOI:http://dx.doi.org/10.12944/CWE.16.1.13
Copy the following to cite this URL:
Olowoyo D. N, Fadairo E. A, Aziza, A. E. Concentration Dependent Effects of Green Inhibitors on Gravimetric Indices of Corrosion Linked Metal Integrity. Curr World Environ 2021;16(1). Available From : https://bit.ly/2Qn8EwP
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2020-06-24 |
|---|---|
| Accepted: | 2021-04-19 |
| Reviewed by: | 
 Sadar Aslam
Sadar Aslam
|
| Second Review by: |

 Mehdi Shahraki
Mehdi Shahraki
|
| Final Approval by: | Dr. M. Rafatullah |
Introduction
Heveabrasiliensis, Pennisetum purpureum and Mangifera indica are common tropical plants known for their fast growing, high productivity and high concentration of phytochemicals respectively. M. indica is known for its fruits rather than its leaves making the leaves constitute wastes as they fall off the tree. P. purpureum, grows as weed, and springs out easily from uncultivated land while H. brasiliensis plants are grown for its latex making the leaves also readily available for use. Corrosion of steel and pipes in the oil and gas industry is a major problem facing the industry. This is because metals have the characteristic of being able to dissolve in aqueous solutions over a long period of time.This dissolution tendency of metal or steel is usually mitigated against by the use of corrosion inhibitors1.The steel and metallic coupons utilized in our present study are familiar materials used in construction of pipes and vessels use for crude storage and storage of other chemicals used in the industry. The common problem with using these materials is their dissolution in acidic solutions which is a common solution of the oil and gas industrial processes2- 4. The use of inhibitors to prevent corrosion and dissolution of steel and metals in theseprocesses is common2- 4. In the past, anti-corrosion substances were attributed as chemical agents either with the ability to resist corrosion completely or substances acting through a mechanism involving changes in material of construction for the specific application or substances that could control corrosion via the creation of a barrier layer between the material cum media to avoid direct contact between the two and finally as substances that act by modification of the corrosion media. Anti-corrosion substances use as corrosion media modifiers are usually phosphates, chromates and silicates. The problem remains with the environmental unfriendly and costly nature of the aforementioned substances. In recent times, there are mounting studies on the use of green inhibitors as anti-corrosion agents5,6. Plants are known to contain certain bioactive principle called phytochemicals which are natural and non-nutritive substances that give protection against external stresses7. Researches on green inhibitors are on the increase because of their non-toxic and eco-friendly effects which is deficient in chemical based anti-corrosion agents.
This study, was therefore designed to analyze the concentration dependent effects of green inhibitors efficiency on weight loss and material strength of laboratory- induced corroded metallic coupons using three different green inhibitors separately on one hand and on the other hand, combined.
Materials and Method
Collection and Identification of Plant Materials
H. brasiliensis, P. purpureum and M. indica leaves were collected fresh during the dry season from communities in Effurun, Delta State, Nigeria. The leaves were subsequently identified and authenticated by a botanist in the Biological Science Section of the Department of Industrial Safety and Environmental Technology, Petroleum Training Institute, Effurun, Delta State, Nigeria. The harvested leaves were thoroughly rinsed with distilled water and left to drain at room temperature, air –dried, pulverized using 9245 Panasonic Blender and stored until ready for use7.
Collection and Identification of Metal Coupons
Carbon steel, iron and aluminum coupons were collected and identified by the Welding and Fabrication Unit of Mechanical Engineering Technology Department of Petroleum Training Institute Effurun, Nigeria.
Determination of Strength of Plant Extracts (Green Inhibitors) used as Green Inhibitors
The leaves of H. brasiliensis, P. purpureum and M. indica (100g) were used for this study. The pulverized leaves were dissolved in deionized water, allowed to stand for 30mins; it was then filtered using size 20 Whatman filter paper. The solid residue of the filtrate was determined by drying 1ml of the filtrate at 200°C in a pre-weighed watch glass. The solid content was found to be 0.80mg/ml8.
A 50% and 100%(v/v) solutions of the extract was prepared by subjecting 50g and 100g of the pulverized leaves in 100ml of deionized water. The mixture was then subjected to control heating using the water bath until a paste like residue was formed. The residue formed was mixed with the 15% HCl in the ratio 4:1 before the insertion of metal coupons9.
Determination of Weight Loss (kg) of Metals (ASTM C694-90a, 2016)10 as Outlined by11
Metal coupons collected from Petroleum Training Institute Welding Workshop were immersed in 15% HCl medium for 2hrs. The coupons were sand papered, rinsed with deionized water and dried in the oven until a constant weight was obtained. They were then allowed to cool in a desiccator and weighed to determined pre-treatment weight (W1). The metal coupons were then immersed in 15% HCl for a maximum of 72hrs without any inhibitor12. Another set of iron aluminum and steel coupons were also immersed in 15% HCl treated with extracts of H. brasiliensis, P. purpureum and M. indica respectively (in the ratio 4:1). At the end of 72hrs, the metal coupons were retrieved from the respective medium, rinsed under running water, placed in an oven at 70oC for 15minutes to dry. The coupons were then placed in a desiccator to cool before determination of final weight (W2) using the Mettle Toledo Weighing Balance (model ELF11/148). This was done according to modified Standard Practice for Preparing, Cleaning and Evaluating Corrosion Test Specimens (SPPCECTS). Theweight loss was determined as the difference between the weight of the coupons before treatment with medium and inhibitor and weight after treatment by 100 as shown below:
weight loss of metal coupons=
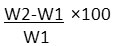
Where:
W2= weight loss in gram after treatment
W1= weight loss in gram before treatment
Determination of material strength
The material strength of the metal was determined as undeformed stress using the compression loading. Hence stress was expressed as:
σ =F/A
Where F= force (N) acting on undeformed area A (m2)
Determination of Corrosion Rate of Metals
Corrosion rate was calculated according to the method outlined by13 assuming uniform corrosion over the entire metal strip surface. The corrosion rate in mil per year (mpy) was calculated by substituting the weight loss of the respective metal in the formula:
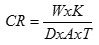
Where:
W= weight loss in grams
K= constant (22, 300)
D=metal density in g/cm3
A= coupon area (cm2)
T= time = (3 days)
Determination of inhibition efficiency of inhibitors
The inhibition efficiency of the inhibitors were calculated using the formula
IEm (%) =
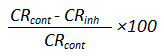
Results
Figures 1 and 2 show the results of the effects of green inhibitors of H. brasiliensis, P. purpureum and M. indica inhibitors at 50% and 100% concentration on material strength of aluminum, iron and steel in 15% HCl as corrosion medium.
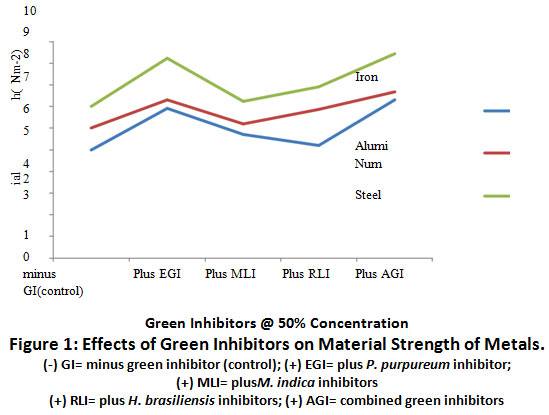 |
Figure 1: Effects of Green Inhibitors on Material Strength of Metals. Click here to view Figure |
The result of the effects of green inhibitors on material strength of metal coupons is represented on Figure 1. The material strength of (+) EGI was significantly elevated (p≤0.05) when compared to (-) GI metal coupons. Combination of the three green inhibitors, (+) AGI, caused a further increase in material strength of metal coupons relative to (+)EGI exposed coupons. Although, the difference was not significant (p≥0.05).
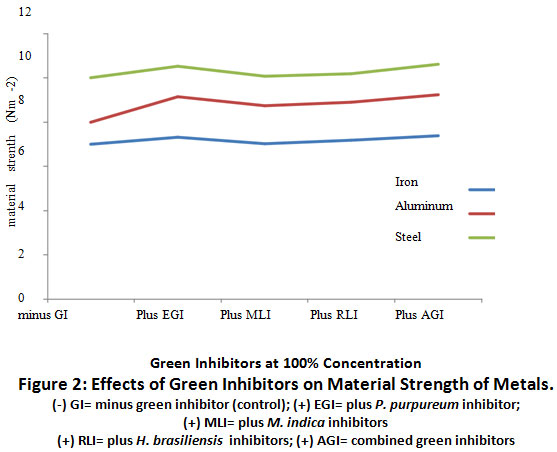 |
Figure 2: Effects of Green Inhibitors on Material Strength of Metals. Click here to view Figure |
The relationship between material strength of iron, aluminum and steel strips at 100% green inhibitors are represented in Figure 2.0. There was a slight decrease (p≥0.05) in the material strength of iron, aluminium and steel in the absence of green inhibitor (-) GI, relative to the material strength of metals when (+)EGI was present at 100% concentration. There was also a slight elevation (p≥0.05) in the material strengths of iron, aluminium and steel when all green inhibitors were combined, (+) AGI, when compared to when they were used individually (+EGI, + MLI and + RLI).
Figures 3 and 4 show the effects of green inhibitors from H.brasiliensis, P. purpureum and M. indica inhibitors at 50 and 100% concentration on weight loss of aluminum, iron and steel in 15% HCl as corrosion medium.
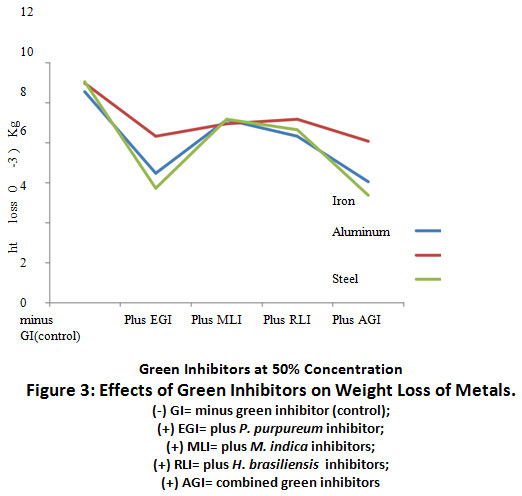 |
Figure 3: Effects of Green Inhibitors on Weight Loss of Metals. Click here to view Figure |
The result of the effects of green inhibitors at 50% concentration on weight loss of metal coupons is represented on Figure 3.0. There was a decrease in weight loss of (+) EGI when compared to (-) GI treated metal coupons. Combined treatment of coupons with (+) AGI caused a further reduction (p≤0.05) in weight loss relative to the control, (-)GI.
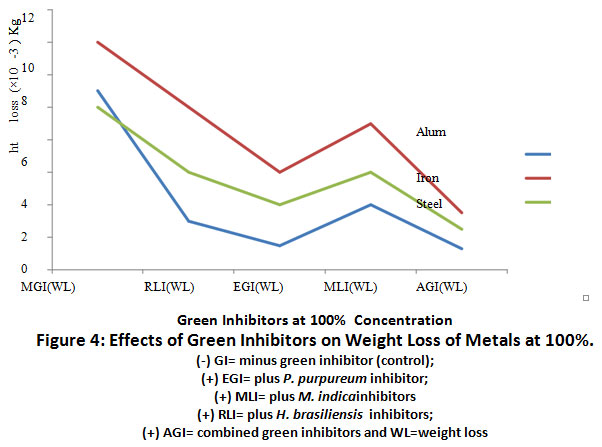 |
Figure 4: Effects of Green Inhibitors on Weight Loss of Metals at 100%. Click here to view Figure |
The result of the effects of green inhibitors at 100% concentration on weight loss of aluminium, iron and steel coupons is represented in Figure 4.0. There was a significant (p≤0.05) decrease in weight loss of (+) EGI - treated aluminium coupon when compared to (-) GI treated coupons. Treatment of iron and steel with (+) EGI showed a decrease in weight loss relative to their corresponding controls, (+) M.indica (WL), and (+) H.brasiliensis(WL).Combined treatment of metal coupons with (+) AGI caused a further reduction (p≤0.05) in weight loss relative to the (+) EGI inhibitor only.
Figure 5 shows the effects of green inhibitors from H.brasiliensis, P. purpureum and M. indicagreen inhibitors at (50 and 100)% concentration as corrosion inhibitors on corrosion rate of aluminum, iron and steel in 15% HCl as corrosion medium.
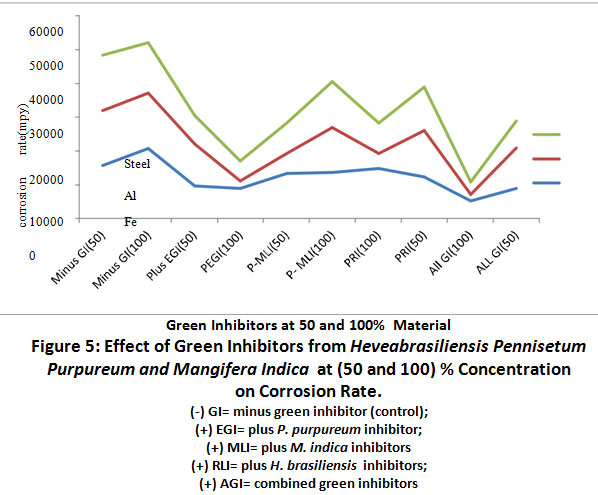 |
Figure 5: Effect of Green Inhibitors from Heveabrasiliensis Pennisetum Purpureum and Mangifera Indica at (50 and 100) % Concentration on Corrosion Rate. Click here to view Figure |
The effects of green inhibitors of H.brasiliensis, P. purpureum and M. indica at (50 and 100) % on corrosion rate of metals are represented in Figure 5.0.Plus P. purpureum inhibitors (plus EGI and PEGI-100) caused a significant (P<0.05)decrease in corrosion rate of aluminium, iron and steel relative to the other green inhibitors,(+)MLI at 100%, (+) PRI at 100%, and +RLI). Combined (+AGI at 100) caused afurther decrease in corrosion rate of iron, aluminium and steel when compared to plusP. purpureum green inhibitor at 100% concentration.
Figures 6 and 7 show the inhibition efficiency of H.brasilienses, P. purpureum and M. indica inhibitors on aluminum, iron and steel in 15% HCl as corrosion medium at (50 and 100)% concentration respectively.
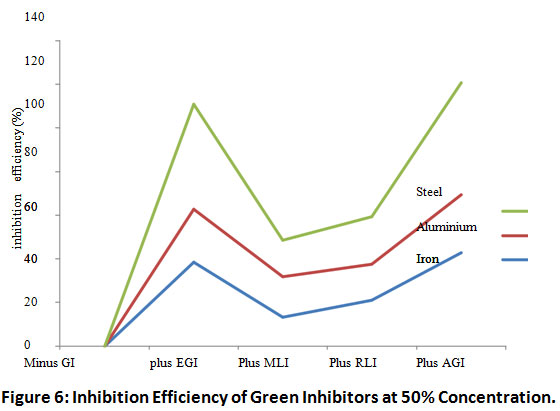 |
Figure 6: Inhibition Efficiency of Green Inhibitors at 50% Concentration. Click here to view Figure |
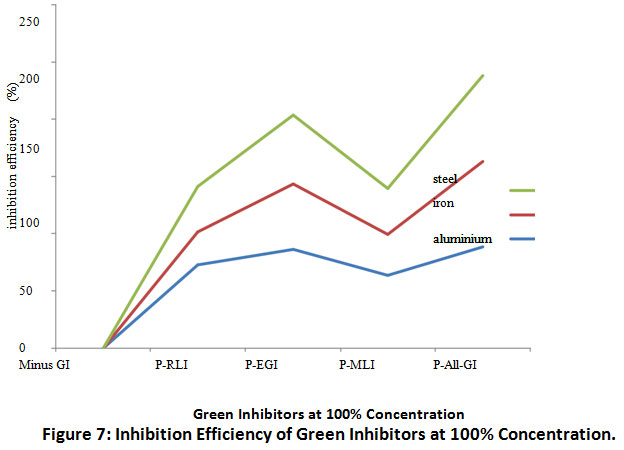 |
Figure 7: Inhibition Efficiency of Green Inhibitors at 100% Concentration. Click here to view Figure |
The results of inhibition efficiency of green inhibitors are represented in Figures 6.0 and 7.0. The inhibition efficiency for plus all inhibitor was 86%, 57% and 60% at 50% concentration for aluminium, iron and steel coupons respectively while treatment of the same medium with combined green inhibitors at 100% concentration caused an increase in the inhibition efficiency to 88% 75% and 74% for aluminium, iron and steel respectively.
Figure 8 shows the relationship between inhibitors efficiency and the weight loss of metal coupons.
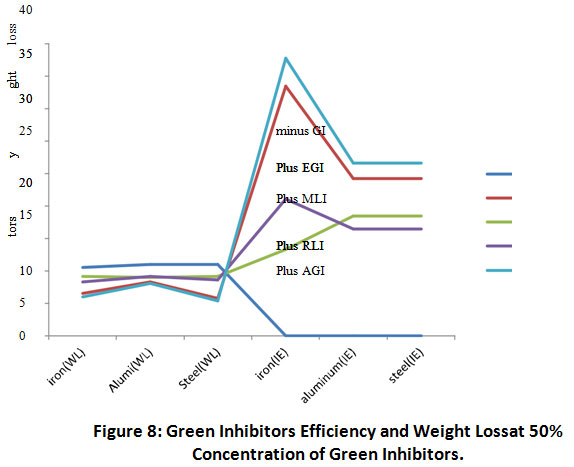 |
Figure 8: Green Inhibitors Efficiency and Weight Loss at 50% Concentration of Green Inhibitors. Click here to view Figure |
The relationship between inhibitor’s efficiency and weight loss of coupons in 15% acid medium is presented in Figure 8. There was a positive correlation between the inhibition efficiency of all green inhibitors (+ AGI) and the low weight losses of iron aluminium and steel treated with (+)AGI. There high inhibition efficiency (p=0.05) of P. purpureum inhibitor treated iron, aluminium and steel correlated directly with the low weight losses of the respective metal coupons.
Discussion
The ability of a given metal or material to withstand stress or strain is usually used as an indicator of metal integrity6. Observation of Figures 1 and 2 showed a general increase in the material strength of metal coupons exposed to the different green inhibitors utilized in this study and an increase in the material strength of elephant grass-coupon treated iron and steel at both 50 and 100 (%) when compared to their controls (minus inhibitor acid exposed metals). This could be due to the ability of the P. purpureum green inhibitor to avert rusting of the metal coupons and consequently sustain coupon integrity. Our finding is in alignment with the report of14.
A review on the anticorrosion tendencies of some phytochemicals in various corrosion medium showed a positive correlation between plant phytochemicals and metallic coupons in all the media studied15, 16. Observation of Figures 1-8 showed slight to significant improvement on the material strength, weight loss, corrosion rate and inhibition efficiency of the metal coupons and green inhibitors examined in this study. Our result is in tandem with the reports of 17- 20
Study showed the tendency of some green inhibitors to reverse corrosion of steel20. In our present study, corrosion of the three metallic coupons was inhibited by the extracts of P.purpureum, H. brasiliensisand M. indica as evident by the enhanced weight and material strength of coupons. Our findings from this study are in tandem with the work of19.
Study also showed that the inhibition efficiency increased with increased inhibitors concentration18. This claim was verified in our present study at higher concentration, 100% green inhibitors concentration, there was increased inhibition efficiency of all metallic coupons relative to the 50% concentration.
The major interest of this study was to determine if there is any possible relationship between the extracts of H. brasiliensis, P. purpureum and M. indica (green inhibitors) on some gravimetry indicators of metal corrosion. The possibility of the green inhibitors examined in this study to inhibit corrosion was established.
Acknowledgement
The authors wish to acknowledge the final year students of the Department of Science Laboratory Technology for their assistance in the Laboratory during the course of the study.
References
- Hosseini SMA., Salari, M., Ghasemi, M. 1-methyl-3-pyridine-2-yl-thiourea as inhibitor for acid corrosion of stainless steel. Mater Corros, 60:963-968 (2009).
CrossRef - Brondel, D. Corrosion in the oil industry-Schlumberger. Journal of Petroleum Technology, 39:756-762 (1987).
CrossRef - Omotioma, M., and Onukwuli, O. D. Evaluation of pawpaw leaves extracts as anti-corrosion agent for aluminium in Hydrolytic acid medium. Nigerian Journal of Technology, 36 (2): 496-504 (2017).
CrossRef - Chew Y.L., Chan, E. W.L, Tan, P.L., Lim, Y. Y., Stanslas, J and Goh, J.K. Assessment of phytochemical content, polyphenolic composition, antioxidant and antibacterial activities of leguminosae medicinal plants in Peninsular Malaysia. ISCMR, 11:12-19 (2011)
CrossRef - Sorkhabi, H.A, Asghari, E. Effect of hydrodynamic conditions on the inhibition performance of 1-methionine as green inhibitor. ElectrochimActa 54:162-167.(2008)
CrossRef - Oguzie, EE. Studies on the inhibitive effect of Ocimumviridis extract on the acid corrosion of mild steel. Mater ChemPhys 99:441-446.(2008)
CrossRef - ASTM International, 100 Barr Harbor Drive, P.O.Box C700, West Conshohocken, P.A, 19428-2959 USA
- Obi, F.O and Fadairo, E.A. Influence of anthocyanin-free aqueous extract of Hibicus sabdariffa L petal on cadmium toxicity in male rats. Nig. J. Life. Sc. 3(1):78-95 (2013).
- Onuegbu, T.U., Umoh, E. T and Ehiedu, C. U. Emilia sonchifolia extract as green corrosion inhibitor for mild steel in acid medium using weight loss method. Journal of Natural Sciences Research, 3(9): 52-55(2013).
- Arockiasamy,P., Sheela, XQR., Thenmozhi, G., Franco, M., Sahayaraj, J.W and Santhi, J.R (2014). Evaluation of corrosion inhibition of mild steel in !M hydrochloric acid solution by Mollugocerviana. https://doi.org/10.1155/2014/679192 (2014)
CrossRef - Lebrina, M Robert, F., Roos, C. Inhibition effect of alkaloids extract from AnnonaSquamosa plant on corrosion of C38 steel in normal hydrochloric acid medium. Int. J. ElectrochemSci, 5:1698-1712.( 2010).
- Afia L., Salghi, R., Bammou, L., Bazzi, LH., Hammouti, B., Bazzi, L.Application of Argan plant extract as green corrosion inhibitor for steel in 1 mol-1HCl. ActaMetall Sin (Eng. Letters) 25:10-18(2012)
- Abiola, O.K., Oforka, N. C., Ehenso, E. E and Nwinuka, N. M. Eco-friendly corrosion inhibitors: The inhibitive action of Detonixragia extract for the corrosion of aluminium in acidic media. Anti- Corrosion Methods and Materials, 54 (4): 219-224 (2007).
CrossRef - Kumar, K and Kailas SV. The role of friction al stir welding tool on material flow and weld formation. Material Science and Engineering, 2008 A 485(1-2), 367-374 (2008).
CrossRef - Phillip, L.L. Buchweishaija, J. Y. N and Mkayula, J. Cashew nut shell liquid(CNSL) as an alternative corrosion inhibitor for carbon steel. Tanzanian Journal of Science.27, 9-19 (2001).
CrossRef - Abiola, O. K and Otaigbe, J. O. E. Adsorption behaviour of i-phenyl-3-methylpyrazol-5-one on mild steel from HCl solution. Int. J. ElectroChem. Sci.(3): 191-198. ( 2008)
- Popoola, L. T. Grema, A.S., Latinwo, G.K., Gatti, B., and Balogun, A.S. Corrosion problems during oil and gas production and its mitigation. International Journal of Industrial Chemistry. 2013; 4(35):1-15.(2013)
CrossRef - Sangeetha, M., Rajendran, S., Sathiyabama.J. andKrishnaveni, A. Inhibition of corrosion of aluminium and its alloys by extracts of green inhibitors. Portugalete Electrochemical acta, 31 (1): 41-52(2013)
CrossRef - Alaneme, K. K., Olusegun, S. J., and Alo, A. W. Corrosion inhibition properties of elephant grass (Penniseteum purpureum) extract and effect on mild steel corrosion in IM HCl solution. Alexandria Engineering Journal, 5(8):224-234. https://dx.doi.org/10.1016/j.aej.2016.03.012 (2016)
CrossRef - Amarpreet, K. K, Namita, K.J,.Kalpana, B and Gurmeet, S. Evaluation of Norfloxacin and ofloxacin as corrosion inhibitors for mild steel in different acids: weight loss data. International Journal of creative Research Thoughts; 6 (1):2320-2882 (2018).






