Water Relations of Two Adjacently Growing Tree Species Shorea Robusta Gaertn-and Pinus Roxburbhii Sarg-in the Lower Himalayan Region
1
Department of Forestry and Environmental Science,
D. S. B. Campus ,
Kumaun University,
Nainital,
India
Corresponding author Email: atewari69@gmail.com
DOI: http://dx.doi.org/10.12944/CWE.15.3.08
Copy the following to cite this article:
Tewari A. Water Relations of Two Adjacently Growing Tree Species Shorea Robusta Gaertn. and Pinus Roxburbhii Sarg. in the Lower Himalayan Region. Curr World Environ 2020; 15(3). DOI:http://dx.doi.org/10.12944/CWE.15.3.08
Copy the following to cite this URL:
Tewari A. Water Relations of Two Adjacently Growing Tree Species Shorea Robusta Gaertn. and Pinus Roxburbhii Sarg. in the Lower Himalayan Region. Curr World Environ 2020; 15(3). Available from: https://bit.ly/32WKZ9v
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 01-06-2020 |
|---|---|
| Accepted: | 11-11-2020 |
| Reviewed by: | 
 A Amarender Reddy
A Amarender Reddy
|
| Second Review by: |

 Hiteshi Tandon
Hiteshi Tandon
|
| Final Approval by: | Dr. Saravanan Pichiah |
Introduction
Of the myriad environmental factors influencing distribution and growth of woody plants, water is of paramount importance and is usually the most limiting throughout the world. This situation exists even though global supplies of water are immense. However, the interaction of air circulation patterns, topography, temperature and edaphic factor result in uneven distribution and availability of moisture. Drought is amongst the most important climatic events which can severely impact natural ecosystems. The natural vegetation of the Himalayan region varies from tropical forests in the foothills to alpine meadows above the timberline along an altitude gradient (300-3500m)1.
The western Himalaya receive concentrated rainfall between end of June to mid of September. This is followed by 8-9 months of prolonged drought which has a severe impact on adaptation of plants to drought and ecosystem processes. 2, 3. The Himalayas are warming at a faster rate than the global average due to global warming 4.The forest ecosystem of Himalaya can be seriously impacted by global climate change. The extent to which plant species will be able to adapt to climate change is a key question of great importance for conservation and resource management. The importance of drought in regulating the distribution of Himalayan species is not very well understood.5.
The water potential measurement at pre dawn is important for estimating plant water status 6 as it indicates how the plant integrates soil water availability and the moisture level at which it begins to develop its daily water deficit. An adequate supply of water is essential to the successful growth, leaf conductance and photosynthesis. Estimation of osmotic adjustment at zero and full turgor is a useful indicator of stress tolerance in trees. The essential factor is plant water relations content and turgor to permit normal functioning of the physiological and biochemical process involved in growth. This is controlled by the relative rates of water absorption and water loss. There are some indirect evidences to indicate, that moisture stress can be severe, that moisture stress differs among communities and the differences among species exist in their ability to cope with drought 7.
In the present study we have tried to compare the water relation parameters of two adjacently growing tree species, a broad leaved dipterocarp sal (Shorea robusta Rox.) and chir pine (Pinus roxburghii Sarg.) a conifer which are two major forest forming species of Himalayan region.
Material and Methods
Study Site
The study site is located between 29°8´ and 29°38´ N latitude and 79°20´and 79°45´E longitude at an elevation of 1370m. The study site is situated on the eastern aspect and the slope angle was of 27°. Five trees of each species growing adjacent to each other, approximately within 2-3 m radius were selected. The trees were young 4-5 m in height and circumference varying between 22 and 37cm. The climate of the study site has a monsoon rainfall pattern, mid June to late September accounting for 75-80% of the annual rainfall. In spite of high annual rainfall early summer (preceding the monsoon period) and winters are relatively dry, generally with <10cm monthly rainfall, and potential evapotranspiration that is often in excess of precipitation2. The annual rainfall is generally between 170-190cm. The mean monthly minimum temperature ranges between 8.8°C in January and 21.2°C in June, and the mean maximum temperature between 12.3°C in February and 29.9°C in June.
Tree Layer Analysis
The tree layer analysis was carried out by placing 10 quadrats of 10x10 meter for trees and 5×5m for saplings and 1×1m for seedlings. The data was expressed as density and total basal area, following 8.
Tree water potential (Ψ), Pressure volume curves and leaf conductance were measured on five representative trees each of both the species across the seasons. Measurements were made on twigs located 2 to 3m from the ground.
Soil Water Potential
Soil waterpotential was measured by Psypro water potential system at two depths of 10cm and 60cm following 9.
Twig Water Potential
Pressure chamber (PMS Instrument Co. model 1000, range 70 bars) was used for the determination of water potential and the development of Pressure-Volume Curves (P-V curves). In this study water potential measurements were made for two years on selected sites and species in different seasons. The water potential (Ψ) was measured at predawn (ΨPD) (5.30- 6.30 A.M.) and in the midday (1.30- 2.30 pm) (ΨMD) following 10, 11 and 12.
Pressure volume curves (PV curves) and Components of water potential
PV curves were prepared to develop a relationship between components of water potential and Relative water content (RWC %). PV curves were prepared following the bench drying method from overnight saturated twigs. From PV curves, the osmotic potential at full turgor (OPf), the osmotic potential at zero turgor (OPz) and RWC% at turgor loss point (RWCz) were determined following 7, 13.
Leaf Conductance
The instrument AP4 type diffusion porometer could not measure the leaf conductance of conifers hence conductance of only S. robusta was measured. Leaf conductance measurements of S. robusta was made seasonally, using AP4 porometer (Delta-T Devices) Data were collected from 03 leaves/ individual on the sunny sides of tree and from approximately similar height, in the morning and afternoon (10.30-11.30 A.M &1.30- 2.30 P.M) following7, 14.
Statistical Analysis
The data were subjected to analysis of variance with a 95% confidence level using SPSS version 2016. Species, seasons and year were the factors used for ANOVA. Correlation coefficient was used for expressing relationship between different variables.
Result
Tree layer analysis
S. robusta and P.roxburghii were the dominant species in the selected site with densities of 212 trees/ha and 141trees/ha respectively. The stand was open and trees were young, consequently the total basal area was small (11.89m2/ha). A few scattered trees of Pyrus pashia and Sapium insigne were present. The high number of saplings (840/ha of S. robusta and 1317/ha of P .roxburghii) indicates both the species were regenerating well in the site despite human disturbance. Seedlings were present but were less than sapling number (Table 1).
Table 1: Tree Layer Analysis of S. Robusta and P. Roxburghii
|
Species |
Growth Stage |
Density (ind./ha) |
Total Basal Area (m2/ha) |
|
Shorea robusta |
Tree |
212 |
5.99 |
|
Sapling |
840 |
0.2016 |
|
|
Seedling |
558 |
2.52 |
|
|
Pinus roxburghii |
Tree |
141 |
5.9 |
|
Sapling |
1327 |
0.5042 |
|
|
Seedling |
698 |
1.39 |
Tree Water Potential
S. robusta trees had pre-dawn Ψpd ranging -0.18±.03 MPa to 0.83±0.01MPa during year one and -0.29±0.01 MPa to -0.67±0.04 MPa during year two. The mid- day Ψmd ranged between -0.25±0.02 MPa and -1.27±0.04 MPa in year one and -0.62±0.03 MPa and -1.18±0.03MPa in year two (Fig. 1). The maximum daily change (ΔΨ= Ψmd - Ψpd) was during the summers of the first year and spring of the second year, the values being 0.55 MPa and 0.51 MPa respectively. The daily change in water potential was negligible during the rainy season (Fig. 1).
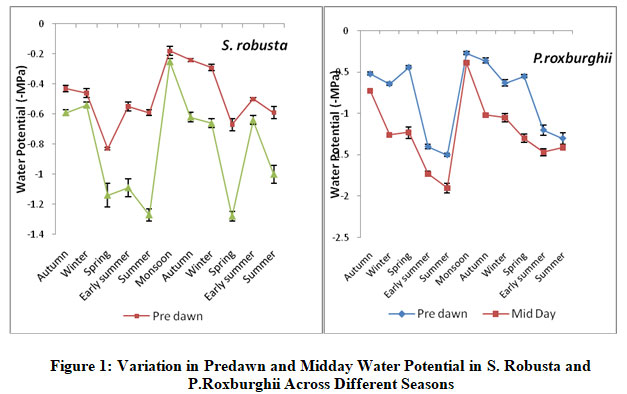 |
Figure 1: Variation in Predawn and Midday Water Potential in S. Robusta and P.Roxburghii Across Different Seasons |
P. roxburghii trees had Ψpd ranging from -0.27±.02 MPa to -1.5±0.02MPa during year one and -0.36±0.03 MPa to -1.3±0.07 MPa during year two. The Ψmd ranged between -0.39±0.01MPa and -1.93±0.06 MPa in year one and -1.02±0.03 MPa and -1.47±0.04 MPa in year two (Fig. 1).The maximum daily change was during the spring season in both the years, the values being 0.79 MPa and 0.75 MPa respectively. The daily change in P roxburbhii was minimal during the peak summer time. The comparison of S. robusta with adjacently rooted P.roxburghii trees indicated that of 11 predawn water potential values 10 were higher for S. robusta. However, T-test between pre dawn water potential of P.roxburghii and S. robusta were not significantly (NS) different.
ANOVA showed that water potential (both Ψpd and Ψmd) varied significantly across species season and year (P<0.01). The interaction between species and year, species and season, year and season were also varies significantly (P<0.01).
Water Potential Components
Using pressure volume (PV Curves) osmotic potential components and relative water conetent (RWC%) of twigs were estimated in four seasons namely monsoon, autumn, winter and early summer season.
The values of osmotic potential at full and zero turgor remained more or less constant for S. robusta from monsoon to winter and then declined during early summer by -0.50MPa (from -1.6 to -2.1MPa) and -0.40 MPa (from -2.5 to -2.9MPa) during the period of maximum osmotic adjustment (winters to early summer) (Table 2)
Table 2: Season Changes in Osmotic Potential at Full Turgor andat Zero Turgor and Relative Water Content at Zero Turgor (RWC%) in S. Robusta and P. Roxburghii. (The Value Represented are Mean of Two Years)
|
Seasons |
Water Potential Components |
||
|
OP Full (MPa) |
OP Zero (MPa) |
RWC (%) |
|
|
S.robusta |
|||
|
Monson |
-1.6 ±0.3 |
-2.5 ±0.1 |
85.7 ±7.5 |
|
Autumn |
-1.7 ±0.2 |
-2.4 ±0.1 |
77.5 ±5.6 |
|
Winter |
-1.7 ±0.2 |
-2.1 ±0.3 |
84.6 ±8.0 |
|
Early summer |
-2.1 ±0.5 |
-2.9 ±0.4 |
80.9 ±10.2 |
|
|
|
|
|
|
P. roxburghii |
|||
|
Monson |
-0.9 ±0.1 |
-1.3 ±0.2 |
86.1 ±8.4 |
|
Autumn |
-1.5 ±0.2 |
-2.6 ±0.6 |
80.0 ±7.6 |
|
Winter |
-1.8 ±0.4 |
-2.7 ±0.3 |
74.3 ±11.2 |
|
Early Summer |
-1.4 ±0.2 |
-1.8 ±0.3 |
75.3 ±10.4 |
P. roxburghii showed a gradual decline in osmotic potential values from monsoon to winter season in osmotic potential at full turgor. The osmotic potential at zero turgor showed a much greater decline of -1.4MPa. The osmotic potential at zero and full turgor showed a rise thereafter in early summer. The osmotic potential at zero turgor values declined from -1.3 to -2.7MPa (Table 2).
In S. robusta and P. roxburghii the values of relative water content (RWC%) were fairly stable. In S. robusta the values ranged between 77.5% and 85.7%. It declined slightly from monsoon to autumn and then rose slightly in winters. In P. roxburghii the values of RWC(%) ranged between 74.3% and 86.1%. The species showed more or less similar seasonal pattern of relative water content. It declined continuously from monsoon to winters and then rose in early summer (Table 2).
ANOVA showed that osmotic potential (at full and zero turgor) and relative water content (%) varied significantly across species and season (P<0.01). The interaction between species and season, also varied significantly (P<0.01).
Soil Water Potential
Across both the years at 10cm depth the soil water potential ranged between -0.38±0.02MPa and -4.80±0.03MPa. At 60cm depth the soil water potential ranged between -0.19±0.01MPa and 2.77±0.02MPa (Fig. 2)
 |
Figure 2: Seasonal Change in Soil Water Potential (-Mpa) at 10cm and 60cm Soil Depth |
In year 1,soil water potential remained high from autumn to spring and then declined conspicuously in early summer to rise sharply thereafter until rainy season. In second year soil water potential declined from rainy to winters, increased during spring, declined moderately during early summer and then increased during later half to summer. The most negative values occurred in early summer in both years (Fig. 2).
ANOVA showed that there was no significant variation in soil water potential across years however, it varied significantly across seasons (P<0.01). The interaction between year and season also varied significantly (P<0.01).
Leaf Conductance
The instrument AP4 type diffusion porometer could not measue the leaf conductance of conifers hence the conductance of only S.robusta was measured. Leaf conductance was measured over five seasons autumn, winter, spring, early summer and summer over a period of two years. Across all the seasons the morning leaf conductance varied between 82.5±6.25 and 563.6±63.8 m mol m-2sec-1. The afternoon conductance varied between 70.63±5.69 and 248.5±5.19 m mol m-2sec-1 (Fig. 3). The morning and afternoon conductance was lowest during early summer of Yr2 and highest at autumn of Yr1. Across all the seasons and years the morning leaf conductance was higher than the afternoon conductance. ANOVA showed that morning leaf conductance and afternoon leaf conductance varied significantly across years and season (P<0.01). The interaction between years and season, was also significant (P<0.01).
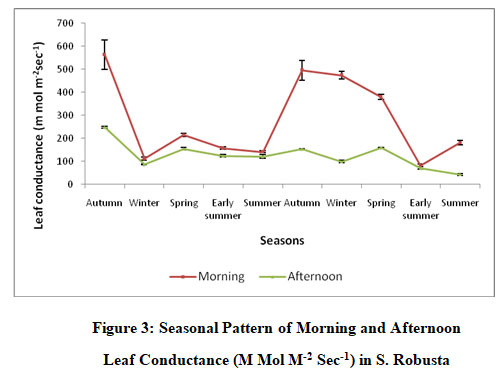 |
Figure 3: Seasonal Pattern of Morning and AfternoonLeaf Conductance (M Mol M-2 Sec-1) in S. Robusta |
Discussion
The study site is characterised by an approximately three-month period of heavy rainfall and warm temperature from mid-June to mid September, and nine months of light rainfall or no rain with long stretches of drought. Moisture conditions of these forests would also be impacted due to global warming and climate change. S. robusta maintained relatively high predawn water potential even in summers when day time temperature was very high. The deeper soils which provide a large soil moisture pool seemed to serve this deep rooted species S robusta well during the summer drought. The pre-dawn shoot water potential of deep rooted species is higher (less negative) than shallow rooted ones as soil water availability is higher at lower depths.15, 7 On the other hand the P. roxburghii showed low predawn water potential during early summer and summer season (-1.4 and -1.5MPa) and the daily change declined with increasing stress (0.33MPa and 0.27MPa) indicating the ability of P. roxburghii to close its stomata as drought intensifies a strategy useful for encroaching in to new sites poor in moisture. The species appears to have a clear strtegy for drought avoidance. Evidently, if adaptation to the existing level of drought were the main determiner of competitive outcome, P. roxburghii would out compete S. robusta. Subsequent to disturbance P. roxburghii is expanding in transition sites where the species comes up at the cost of broadleafed species.2 In S. robusta winter to spring time rise (-0.46 to -0.83 MPa) in tree water potential was a pronounced feature which coincided with the timing of maximum phenological activities. Similar results was also observed by 12 in low altitudinal species of Himalayan region. The daily change in P.roxburghii which indirectly represents ability to keep stomata open and conduct water freely, tended to decrease in dry season.. The summer time decline in predawn water potential was sgnificant in P. roxburghii and absent in S. robusta. The deep roots and deep soil seem to explain the stable predawn water potential of sal.
The conductivity of leaf surface to water vapour is an important integrator of the plant water conditions.16 Daily pattern of leaf conductance vary along environmental gradients17 and with succession.18 There was a significant decrease in leaf conductance during the peak summer time in S. robusta. According to 19 the upper canopy species have maximum mean conductance 294 m mol m-2 sec-1 which is slightle higher than that of 211 m mol m-2 sec-1 for seasonal tropical forests. In the present study the maximum conductance of S. robusta was 563.6 m mol m-2 sec-1 which is marginally higher that the conductance of Hopea ferrea (510 m mol m-2 sec-1) by 20 . Leaf conductance appeared to be related to leaf development, because it decreased in early summer and in summer, when leaves expanded and matured. After replenishment of soil water during the rainy season leaf conductance was high in autumn. Similar results were also observed by 21.
Acknowledgement
I would like to thank Head. Department of Forestry & Environmental Science, D.S.B. Campus, Kumaun University, Nainital for providing laboratory facilities.
Conflict of Interest
The authors do not have any conflict of interest.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conclusion
It is difficult to explain species distribution on the basis of their adaptation to water stress alone. Several other environmental factors may contribute to their regeneration and distribution22-25. S. robusta was not subjected to severe water stress across all seasons. Sal is known to repeatedly die-back at seedling stage when the tap roots are unable to penetrate into deep soil with favourable water conditions. is quite likely that it is affected by water deficiencies only at seedling/ sapling stages and once its root system is fully entrenched into deeper soils it is not subjcted to water stress. The shallow rooted P. roxburghii avoids severe water stress by closing stomata when water stress is high particularly during the summer drought.
References:
- Poudyal K, Jha P. K, Zobel D. B, Thapa C.B. Patterns of leaf conductance and water potential of five Himalayan tree species. Tree Physiol. 2004; 24:689-699.
CrossRef - Singh, J.S, Singh, S.P. Forests of Himalaya. Nainital,Gyanodaya Prakashan, India, 1992.
- Zobel, D.B, Singh, S.P. Himalayan forests and ecological generalization. BioScience.1997; 47:735-745.
CrossRef - Yao Y, Zhang B. The mass elevation effect of the Tibetan Plateau and its implications for alpine treelines. Int J Climatol. 2012; 35:1833–1846.
CrossRef - Zobel, D. B, Singh, S.P. Tree water relations along the vegetational gradient in the Himalayas. Curr. Sci. 1995; 68:742-745.
- Zlatev, Z, Lidon F.C. An overview on drought induced changes in plant growth, water relations and photosynthesis. Emirates Journal of Food and Agriculture. 2012; 24(1): 57-72.
CrossRef - Tewari A.Timing of drought effects on water relation of certain major forest types of lower and middle central Himalaya. Ph.D. Thesis, Kumaun University, Nainital; 1998.
- Saxena, A.K, Singh, J.S. A phytosociological analysis of woody species in forest communities of a part of Kumaun Himalaya. Vegetatio, 1982; 50:3-22.
CrossRef - Singh S. P, Zobel D.B, Garkoti S. C, Tewari A, Negi C.M.S. Pattern in water relations of central Himalayan trees. Trop Ecol. 2006; 47:159-182.
- Tewari A. Tree water relation study in Sal (Shorea robusta Gaertn) forests in Kumaun Central Himalaya. J Environ Biol. 1999; 20(4):351-355.
- Zobel D. B, Garkoti S. C, Singh S.P, Tewari A, Negi C.M.S. Patterns of water potential amongst forest types of the central Himalaya. Curr Sci. 2001; 80:774-779.
- Tewari A, Shah S, Singh N, Mittal A. Treeline species in Western Himalaya are not water stressed: a comparison with low elevation species. Trop Ecol. 2001; 59(2):313–325.
- Pallardy S. D, Pereira J. S, Parker W.C. Measuring the state of water in tree systems. IN: Lassoie JP, Hinckley TM (eds), Techniques and Approaches in Forest Tree Ecophysiology. CRC Press, Boca Raton, FL. 1991; 27-76.
- Garkoti S.C, Zobel D.B, Singh S.P. Comparision of water relations of seedlings and trees of two Himalayan Oaks. International Journal of Ecology and Environmental Science. 2000; 26:213-222.
- Abrams M.D. Adaptations and responses to drought in Quercus species of North America. Tree Physiol. 1990; 7: 227-238.
CrossRef - Smith W.K, Hollinger D. Y. Measuring stomatal behavior. In: J.P. Lassoie and T.M. Hinckley (eds), Techniques and Approaches in Ecophysiology. CRC Press Boca Raton FL. 1991; 141-174.
- Korner C. Mayr R. Stomatal behavior in alpine plants communities between 600 and 2600meters above sea level. Ch. 11 IN Grace, J.E.D., Frod and P.G. Jarvis (eds), Plants and their Atmospheric Environment. Blackwell, Oxford. 1981.
- Lassoie J.P, Hinckley T.M, Grier C.C. Coniferous forest of the Pacific Northwest Ch.6. IN Chabot, B.F. and H.A. Mooney (eds), Physiological Ecology of North American Plant Communities. Chapman and Hall, New York. 1985.
CrossRef - Kramer P.J, Boyer J.S. Water relations of plants and soils. San Diego: Academic Press. San Diego. 1995.
CrossRef - Pitman I. J. Ecophysiology of tropical dry evergreen forest. Thailand measured and modeled stomatal conductance of Hopea ferrea, a dominant canopy emergent. Journal of Applied Ecology.1996; 33: 1366-1378.
CrossRef - Garkoti S.C, Zobel, D.B, Singh, S.P. Variation in drought response of sal (Shorea robusta) seedlings. Tree Physiol.2003; 23:1021-1030.
CrossRef - Yadav,SS, N Longnecker, F Dusunceli, G Bejiga, M Yadav, AH Rizvi, M Manohar, AA Reddy, Z Xaxiao, Weidong Chen(2007) 4 Uses, Consumption and Utilization (ed. S.S. Yadav) Chickpea Breeding and Management, 101-142, CAB International, Wallingford, Oxfordshire, England.
- Reddy A Amarender, Parthasarathy Rao P, Yadav OP, Singh IP, Ardeshna NJ, Kundu KK, Gupta SK, Rajan Sharma, Sawargaonkar G, Dharm Pal Malik, Shyam Moses D and Sammi Reddy K. (2013). Prospects for kharif (Rainy Season) and Summer Pearl Millet in Western India. Working Paper Series no. 36. Patancheru 502 324, Andhra Pradesh, India:International Crops Research Institute for the Semi-Arid Tropics. 24 pp.
- Reddy AA (2013)Agricultural productivity growth in Orissa, India: Crop diversification to pulses, oilseeds and other high value crops, African Journal of Agricultural Research 8 (19), 2272-2284.
CrossRef - Reddy AA(2013a) Strategies for reducing mismatch between demand and supply of grain legumes, Indian Journal of Agricultural Sciences 83 (3), 243-59.






