Influence of Incubation Periods on Extractable Fluoride and Phosphorous at Different Exchangeable Sodium Percentage Levels
DOI: http://dx.doi.org/10.12944/CWE.12.3.23
Copy the following to cite this article:
Barnwal M. K, Sharma S. K, Bhat M. A, Rani S. Influence of Incubation Periods on Extractable Fluoride and Phosphorous at Different Exchangeable Sodium Percentage Levels. Curr World Environ 2017;12(3). DOI:http://dx.doi.org/10.12944/CWE.12.3.23
Copy the following to cite this URL:
Barnwal M. K, Sharma S. K, Bhat M. A, Rani S. Influence of Incubation Periods on Extractable Fluoride and Phosphorous at Different Exchangeable Sodium Percentage Levels. Curr World Environ 2017;12(3). Available from: http://www.cwejournal.org/?p=184
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2017-05-25 |
|---|---|
| Accepted: | 2017-11-06 |
Introduction
Fluoride (F−) is an anion of fluorine characterized by small radius having extraordinary tendency to act as a ligand and readiness to form numerous organic and inorganic compounds in soil, rocks, air, plants and animals.1 The element fluorine is present in phosphatic fertilizers, soils and plants. The concentration of this element in these resources, although variable, is of the order 3×104, 3×102 and 3×100 ppm, respectively; therefore, decreases by a magnitude of two at each level in the sequence: fertilizer-plant-soil.2 Fluorine can substitute hydroxyl ions in different minerals and, therefore, forms fluorapatite (Ca10(PO4)6F2), which is a common fluorine mineral in the sediments and soils.3 In addition, there are other minerals, for instance: CaF2, AlF3 and Al2(SiF6)2, as fluorine has the ability to form complex ions with aluminum (AlF2+, AlF2+, AlF4-), thus, it can control the activity of Al3+-ions in a soil solution. Parent material determines the concentration of fluorine in different soils; however, its distribution in soil profile is ascertained by the rate of mineral decomposition, pH and proportion of the clay fraction.4 Jakovljevic et al.3 substantiated that a close relationship exists between fluorine and phosphate in both primary and secondary minerals. Chhabra et al5 evinced that increase in soil ESP and pH resulted in increased water extractable F. As the application of fluorine is enhanced, the water extractable fluorine in the soil is increased; however, the relative increment is greater in soil having high ESP coupled with high pH.
Table 1: Physico-chemical characteristics of the experimental soil
|
Characteristics |
Content |
References |
|
Mechanical composition (%) Sand Silt Clay Texture |
71.70 18.96 9.34 Sandy loam |
Piper [11] |
|
pH(1:2) |
8.18 |
Richards [12] |
|
Electrical conductivity(1:2) [dS/m] |
0.95 |
Richards [12] |
|
Cation exchange capacity(CEC) [cmol(+)/kg] |
10 |
Richards [12] |
|
Saturation percentage |
38 |
Richards [12] |
|
Organic carbon [%] |
0.42 |
Walkley and Black [13] |
|
Olsen’ s extractable-P [mg/kg soil] |
3.45 |
Olsen et al.[14] |
|
Water extractable-F [mg/kg soil] |
4.25 |
Brewer [15] |
|
Exchangeable sodium percentage |
6.2 |
Richards [12] |
 |
|
 |
|
Since, Phospho-gypsum a by-product of various phosphatic fertilizer industries finds its extensive use in the reclamation of sodic soils in India especially Haryana as it contains more than 90 per cent calcium sulphate. On the contrary, it contains large quantities of fluoride varying from 2-4 per cent [6]. The excessive amount of fluoride is governed by its concentration in equilibrium solution.6,7 The fluoride concentration in equilibrium solution is governed by a number of factors such as pH, organic carbon, ESP, calcium carbonate, texture, parent material, quality of irrigation water, fertility status and addition of fluoride bearing compounds. Though, water soluble fluoride exclusively cannot control uptake of fluoride by plants but like phosphate and some other anions, is to a great degree adsorbed by soil fabric and its uptake have to be regulated by the quantity-intensity ratio.2 Kaloi et al.8 reported that phosphate release increased in soils having a smaller amount of clay and higher inherent phosphate in conjunction with increasing initial phosphate levels and diminished in soils comprising of higher clay content in addition to increasing incubation period and number of extractions. Rajput et al.9 ascertained that increase in applied P during incubation period resulted in significantly higher P sorption in both soil series (Rustam and Miani). Bhardwaj10 studied the effect of incubation on the extractability of F and P and reported that the extractability of F and P decreased with increase in incubation period. Since, little work has been done on incubation studies of fluoride and phosphorous in sodic soils, therefore, this study was attempted to assess the effect of incubation periods on the extractable fluoride and phosphorous under different ESP levels.
Materials and Methods
An incubation experiment was conducted in controlled laboratory conditions in the Department of Soil Science, CCS Haryana Agricultural University, Hisar located at 29° 05ꞌ N latitude 75° 38ꞌ E longitude and an altitude of 222 m above mean sea level. The climate of the region is semi-arid subtropical having cold winters and hot summers with minimum temperature nearly zero and maximum temperature around 47℃ and total annual rainfall is450 mm approximately with maximum precipitation during the months of July to September. Bulk surface soil sample (0-15 cm) was collected from village Balsamand (Balsamand Series), District Hisar. The samples were air-dried, thoroughly ground and passed through 2 mm sieve. After mixing properly, the soil was used for laboratory studies. The physico-chemical characteristics of soil are presented in Table 1. The laboratory experiment was planned in Factorial Completely Randomized Design (CRD) having three replications with two levels each of fluoride (40 and 160 mg/kg) and phosphorous (12.5 and 50 mg/kg) and four levels of ESP (6.2, 27.1, 43.7 and 54.9). The soils of different ESP levels (30, 45 and 60) were prepared by applying different amount of sodium bicarbonate dissolved in distilled water to the calculated volume of saturation percentage of the soil. The observed ESP of the prepared soil was 27.1, 43.7 and 54.9. About 50 g of air dried soils of different ESP levels viz. 6.2, 27.1, 43.7 and 54.9 were taken in wide mouthed plastic bottles. The required volume of sodium fluoride (NaF) and potassium dihydrogen phosphate (KH2PO4) solutions were added to obtain fluoride levels (40 and 160 mg/kg) and phosphorus levels (12.5 and 50 mg/kg), respectively. These bottles were kept in BOD incubator at 25±2 ℃ and moisture was well kept at the field capacity by addition of distilled water as and when required. At the end of the required incubation period viz., 1, 3, 7, 14, 21, 28 and 35 days, the soil samples were dried, sieved and analysed for Water extractable fluoride (henceforth, WEF) and Olsen’s extractable phosphorus (henceforth, OEP). Statistical analysis was accomplished with IRRISTAT data analysis package (IRRI) using analysis of variance (ANOVA) for Factorial Completely Randomised Design. The means were compared using least significant difference (LSD) at (p ≤ 0.05) probability level [16].
 |
|
 |
|
 |
|
Results and Discussion
Water extractable fluoride
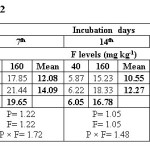
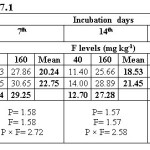
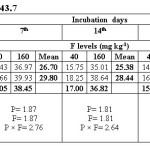
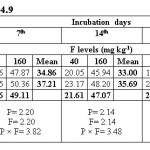
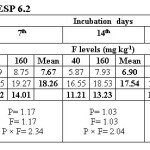
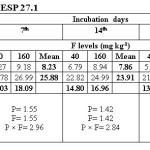
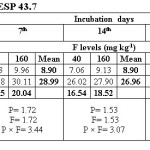
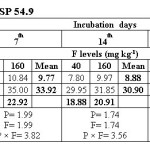
Water extractable fluoride (WEF) significantly increased with increasing fluoride levels in soils at different exchangeable sodium percentage (ESP) levels irrespective of incubation period and this trend was observed at all ESP levels and incubation periods. The WEF observed at 1st day of incubation was 9.04 and 26.80 mg/kg soil whereas it was 4.99 and 9.32 mg/kg at 35th day of incubation at ESP 6.2 and F level of 40 and 160 mg/kg soil (Table 2). With increase in incubation period from 1 to 35 days the WEF significantly decreased from 9.04 to 4.99 mg/kg at applied F level of 40 mg/kg soil at an ESP of 6.2. The per cent decrease in WEF was 44.8 with increasing incubation period from 1 to 35 days in soil having ESP 6.2. Water extractable fluoride increased from 16.09 to 34.78 mg/kg at an ESP level of 27.1 at F levels of 40 and 160 mg/kg soil on first day of incubation period (Table 3). The WEF was 21.38 and 43.58 mg/kg on 1st day of incubation at 40 and 160 mg F per kg soil, respectively at ESP 43.7 (Table 4). Water extractable fluoride decreased from 16.09 to 7.92 mg/kg soil at applied fluoride level of 40 mg/kg soil at ESP 27.1 with increase in incubation from 1 to 35 days and the decrease was 50.8 per cent. However, it decreased from 21.38 to 12.35 mg/kg soil at fluoride level of 40 mg/kg soil and ESP 43.7 with increase in incubation from 1 to 35 days and the decrease was 42.2 per cent. Moreover, the WEF was 27.18 and 53.52 mg/kg soil at ESP 54.9 with increasing application of F at 40 and 160 mg/kg soil at first day of incubation. It decreased from 27.18 to 18.14 mg/kg soil at fluoride level of 40 mg/kg soil and ESP 54.9 with increase in incubation from 1 to 35 days and the decrease was 33.3 per cent (Table 5).
The data indicates that WEF also increased with application of phosphorus. It increased from 16.73 to 19.10 mg/kg with increasing levels of P from 12.5 to 50 mg/kg soil at 1st day of incubation and ESP 6.2 (Table 2). However, it decreased from 16.73 to 6.80 mg/kg soil at applied P level of 12.5 mg/kg soil with increasing incubation from 1 to 35 days at ESP 6.2 and the decrease was 59.4 per cent. At ESP 27.1 and 43.7, the WEF increased with increasing application of P. It increased from 24.11 to 26.75 mg/kg and 30.68 to 34.28 mg/kg at ESP levels of 27.1 and 43.7, respectively at 1st day of incubation with increase in P from 12.5 to 50 mg/kg soil (Table 3 and 4). Water extractable fluoride also increased from 38.73 to 41.97 mg/kg at ESP 54.9 with increasing levels of P from 12.5 to 50 mg/kg soil (Table 5). However, it decreased from 38.73 to 27.93 mg/kg soil at P 12.5 mg/kg soil with increasing incubation from 1 to 35 days at ESP 54.9 and the decrease in WEF was 27.9 per cent. The data clearly indicate that the higher WEF remains in soluble phase in soils of higher ESP compared to normal or non-sodic soils. It is evident from incubation studies that addition of P in F contaminated soils does not cause any decrease in F extractability, rather it will increase the same. Increasing extractability of water extractable F with increase in applied P levels could be ascribed to the possible exchange reaction between the two anions i.e. F and P. Singh et al.5 also observed that significant increase in the water extractable fluoride with its increasing application in soil. The results are in agreement with those of Bhardwaj.10 The higher extractability of fluoride in soils of higher pH may probably be due to the presence of NaF which has higher solubility than other F compounds in soil [5]. Jakovljevic et al.3 reported that at acidic and neutral reaction (pH 6-7) the adsorption of fluoride is maximum; therefore, with increase in pH, ESP extractable fluoride concentration should increase. Hence, relatively much lower amount of added F would be immobilized in soils of higher ESP as compared to non-sodic soils (ESP 6.2). Larsen and Widdowson2 reported that the higher extractable F at high pH is due to hydroxyl ion replacement. The immobilization of added fluoride in normal soil could be attributed to its conversion or transformation to relatively less water soluble compounds such as CaF2. Thus, the extractability of F in soils is much more dependent on the calcium saturation of the exchange complex. Increasing extractability of water extractable F with increase in applied P levels could be ascribed to the possible exchange reaction between the two anions i.e. F and P.
 |
|
 |
|
 |
|
Olsen’s extractable phosphorus
Olsen’s extractable phosphorus (OEP) significantly increased from 8.42 to 23.38 mg/kg with increase in level of P from 12.5 to 50 mg/kg soil at first day of incubation in soil of ESP 6.2 and the increase was 178 per cent (Table 6). However, it decreased from 8.42 to 5.25 mg/kg with increasing incubation from 1 to 35 days at P level of 12.5 mg/kg soil at ESP 6.2 and the per cent decrease was 37.6. At ESP levels of 27.1 and 43.7 the OEP increased from 9.57 to 27.77 mg/kg and 10.29 to 31.71 mg/kg, respectively with increase in phosphorus from 12.5 to 50 mg/kg soil at first day of incubation and the per cent increase was 190 and 208 at ESP levels of 27.1 and 43.7, respectively (Table 7 and 8). However, with increase in incubation from 1 to 35 days, OEP decreased from 9.57 to 5.41 mg/kg soil and 10.29 to 5.94 mg/kg at ESP 27.1 and 43.7, respectively at P 12.5 mg/kg soil. The per cent decrease in OEP was 43.5 and 42.3 at ESP levels of 27.1 and 43.7, respectively with increase in incubation from 1 to 35 days at P 12.5 mg/kg soil. The OEP decreased from 38.75 to 20.16 mg/kg in soil of ESP 54.9 with increase in incubation from 1 to 35 days at P 50 mg/kg soil. The per cent decrease was 47.9 at ESP 54.9 with increasing days of incubation from 1st to 35th days (Table 9). It is evident from the data that the OEP decreased with increase in incubation irrespective of ESP levels. However, OEP increased significantly with increasing levels of ESP. Furthermore, immobilization of phosphorus was comparatively more in soil of higher ESP compared to normal soil and, therefore, P extractability was more at higher ESP compared to normal soil. Singh et al.5 reported that the impact of phosphorous on soil fluorine was more pronounced at lower ESP compared to higher ESP which might be due to high solubility of P at high ESP thereby reducing its ability to release fluoride through anion replacement. The results are in agreement with those of Bhardwaj.10
Increasing levels of fluoride also resulted in increase in extractability of OEP in soil irrespective of the incubation periods and ESP levels. The OEP increased from 14.73 to 17.06 mg/kg soil with increase in fluoride from 40 to 160 mg/kg soil at ESP 6.2 on 1st day of incubation and it was 9.00 to 11.05 mg/kg soil at 35th day of incubation (Table 6). Olsen’s extractable P observed was 10.99 and 12.93 mg/kg at ESP 27.1 and 11.27 and 13.38 mg/kg soil at ESP 43.7 with increase in fluoride from 40 to 160 mg/kg soil at 35th days of incubation (Table 7 and 8). The OEP was 11.87 and 14.29 mg/kg soil with increase of fluoride from 40 to 160 mg/kg soil in soil of ESP 54.9 at 35th day of incubation (Table 9). The per cent increase was 20.4 at ESP 54.9 with increasing levels of F from 40 to 160 mg/kg soil at 35th day of incubation. Similar results were also reported by Bhardwaj.10 In soil of high ESP, the extractability of P was more which may be ascribed to the presence of sodium phosphate which has higher solubility and so is not available to the plants. Increasing levels of F has also resulted in increased extractability of phosphorus as Olsen’s extractable P in soils of all the ESP levels and also all the incubation periods. The interaction between F and P was statistically significant in relation to extractability of P in soils of all the ESP levels. Increasing levels of F significantly enhanced extractability of P in soils. The higher extractability of phosphorus in soils of higher ESP levels due to increasing levels of fluoride may be attributed to the possible anion exchange reaction between the two anions i.e. fluoride and phosphate.5
From the results of the study, it is concluded that water extractable fluoride (WEF) in soil increased with increasing levels of P and F application together with increased ESP levels. Also, Olsen’s extractable phosphorous (OEP) increased with increasing levels of F, P and ESP. Both water extractable fluoride and Olsen’s extractable phosphorus decreased with increasing days of incubation periods. Hence, the interaction between fluoride and phosphorous is significant with respect to exchangeable sodium percentage.
Acknowledgment
The authors are thankful to the Department of Soil Science, CCS Haryana Agricultural University, Hisar for providing the financial support to complete the experiment.
References
- Habuda-Stanic, M., Ravancic, M. E., & Flanagan, A., A Review on Adsorption of Fluoride from Aqueous Solution. Materials, 7: 6317-6366 (2014).
- Larsen, S., & Widdowson, A. E., Soil fluorine. Journal of Soil Science, 22: 210-221 (1971).
- Jakovljevic., M., Blagojevic, S., & Antic-Mladenovic, S., Fluorine Content in Soils of Northern Pomoravlje. Journal of Agricultural Sciences, 47(2): 121-128 (2002).
- Omueti , J. A. I., & Jones, R. L., Fluorine distribution with depth in relation to profile development in Illinois, Soil Science Society of America Journal, 44: 247 (1980).
- Chhabra. R., Singh, A. & Abrol, I.P., Fluorine in sodic soil. Journal of Soil Science Society of America, 44: 33-36 (1980).
- Singh, A., Chhabra, R., & Abrol, I. P., Effect of Fluorine and Phosphorus Application to a Sodic Soil and their Availability in Soil, Yield and Chemical Composition of Wheat. Soil Science, 128 (2): 90-97 (1979).
- Leone, I. A., Brennan, S. G. and Robbins, W. R. Some Effects of Fluorine on Peach, Tomato and Buck wheat when absorbed through the Roots. Soil Science, 66: 249-266 (1948).
- Kaloi, G. M., Bhughio, N., Panhwar, R. N., Junejo, S., Mari, A. H., & Bhutto, M. A., Influence of Incubation Period on Phosphate Release in two Soils of District Hyderabad. The Journal of Animal & Plant Sciences, 21(4):665-670 (2011).
- Rajput, A., Panhwar, Q.A., Naher, U.A., Rajput, S., Hossain, E., & Shamshuddin, J., Influence of Incubation Period, Temperature and Different Phosphate Levels on Phosphate Adsorption in Soil. American Journal of Agricultural and Biological Sciences, 9 (2): 251-260 (2014).
- Bhardwaj, S., Effect of Fluorine and Phosphorus on the Growth and Chemical Composition of Wheat in Sodic Soils. M.Sc. thesis, CCS Haryana Agricultural University, Hisar, Haryana (2006).
- Piper, C.S., Soil and Plant Analysis. International Science Publishers Corporation, New York (1950).
- Richards, L. A., Diagnosis and Improvement of Saline and Alkali Soils. USDA Handbook No. 60 (1954).
- Walkley, A. J., & Black, C. A., Estimation of Soil Organic Carbon by the Chromic Acid Titration Method. Soil Science, 37: 29-38 (1934).
- Olsen, S. R., Cole, C. V., Watanabe, F. S., & Dean, L.A., Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate. USDA Circular 939 (1954).
- Brewer, R. F., Methods of Soil Analysis. Part 2. Edited of C.A. Black. American Society of Agronomy, Wisconsin, USA. Fluorine, Chapter No. 82: 1135-1148 (1965).
- International Rice Research Institute (IRRI). IRRISTAT for window (CD-ROM) version 4.02b. Los Banos, Philippines, IRRI (2000).







