Biotransformation Studies on Organochlorine Insecticide, Endosulfan by Indigenous Bacterial Isolate
M. Supreeth 1 * and N. S. Raju 1
1
Department of Studies in Environmental Science,
University of Mysore,
Mysuru,
570006
India
DOI: http://dx.doi.org/10.12944/CWE.12.2.20
Aerial application of persistent, bioaccumulative organochlorine pesticide endosulfan on cashew plantations to protect it from mosquito bug has led to contamination of soil and water environments in several parts of South Canara region, India. Endosulfan and its toxic residues like endosulfan sulfate are posing several threats to non-target organisms including humans. Biotransformation of toxic compounds using indigenous microbial strains is considered as safe and cost effective technique in bioremediation. In the present work, the bacterial strain designated as ES-1, has been isolated from the soil by enrichment method. The bacterial strain was found to mineralize endosulfan ˃99% of 100 mg/l completely biotically after 14 days of incubation by forming unknown polar metabolites.Whereas, abiotic degradation resulted in formation of a toxic compound, endosulfan sulfate. Based on 16s rDNA sequence analysis, the strain ES-1 showed 99% similarity to Bacillus sp. The results from the work suggest that, this bacterial strain could be employed for remediation of endosulfan contaminated environments.
Copy the following to cite this article:
Supreeth M, Raju N. S. Biotransformation Studies on Organochlorine Insecticide, Endosulfan by Indigenous Bacterial Isolate. Curr World Environ 2017;12(2). DOI:http://dx.doi.org/10.12944/CWE.12.2.20
Copy the following to cite this URL:
Supreeth M, Raju N. S. Biotransformation Studies on Organochlorine Insecticide, Endosulfan by Indigenous Bacterial Isolate. Curr World Environ 2017;12(2). Available from: http://www.cwejournal.org/?p=17481
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2017-07-09 |
|---|---|
| Accepted: | 2017-08-09 |
Introduction
Pesticides used to protect crops have led to increased crop yield in the modern world. However, unscientific use of various types of pesticides as affected various forms of life in the environment directly and indirectly. Persistent Organochlorine Pesticide (OCPs) has been applied continuously in the last century to improve the agricultural productivity. These OCPs being persistent organic pollutants and their metabolites are still present in the environments which are having mutagenic and carcinogenic effects.1
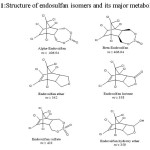
Endosulfan (6,7,8,9,10,10-hexachloro-1,5,5a,6,9,9a-hexahydro-6,9-methano-2,4,3-benzodio-3-oxide) is first generation Organochlorine pesticide introduced in 1950’s and approved for use in 1954 in USA. It exists in two isomers as α-Endosulfan or Endosulfan (I) and β-Endosulfan or Endosulfan (II) in the ratio 7:3 having almost similar insecticidal activities, but have significant differences with respect to their physicochemical properties.2-4 It is a broad spectrum insecticide used on fruit, vegetables, cotton and Ornamental plants to control white flies, aphids, leafhoppers, potato beetles, moth larvae and cabbage worms.5 Endosulfan and its toxic residue endosulfan sulfate are known for Bioaccumulation which can get into the food chain in many ways. The two isomers and other metabolites of endosulfan are shown in the Fig.1.
 |
|
Endosulfan is listed in persistent organic pollutants (POPs) by Stockholm convention in 2011, because of its high toxicity and bioaccumulative nature to most living organisms and was demonstrated to transport from its original source to long distance in the environment, affecting remote human and wildlife population through mammalian gonadal toxicity, genotoxicity and neurotoxicity.6-7 India was a supplier of 70% endosulfan of a market value around $300 million and was the largest consumer of it by consuming 9,000 tonnes every year by its 75 million farmers, making it has world’s largest consumer. Due to its toxicity to humans and other non target organisms, it has been banned in India since 2011 for its production, use & sale, all over India. Due to its Hydrophobic, persistent and bioaccumable nature, the endosulfan strongly bounds to soil and their residues will remain in soil for longer periods.8 Earlier studies have shown the presence of Endosulfan isomers and endosulfan sulfate in various agricultural soils, water and other environmental samples in India and around the world.9-13 These findings show the contamination of various environmental samples with endosulfan and its metabolites along with other pesticides.
Endosulfan was applied onto cashew trees to protect them from mosquito bug in many villages of Puttur, Belthangady, Sullia and Bantwal Taluks of Dakshina Kannada District, Karnataka, India. More than thirty six thousand liters of endosulfan was aerially applied in eight fifty (850) hectares of cashew plantations in the District and also with addition of eleven thousand liters by manual application according to Karnataka cashew development corporation ltd from the year 1980-2000. The applied insecticide remains in soil for longer periods. The half-life of endosulfan is considered more than hundred years.14
Bioremediation or Bioaugmentation is an ecofriendly method in removing the toxic contaminants using microorganisms. Some of the studies which have reported on biodegradation of endosulfan by using microorganisms are.15-23 Bacteria and fungi are considered as a potential candidate in removing contaminants has they use it for their metabolism.24 Indigenous Bacteria and fungi capable of removing toxicants from contaminated soils acts as an excellent bioaugmenting agent and also they do not pose any serious threat to other native flora and fauna.25 In this regard, the present study was carried out to isolate indigenous bacteria capable of degradingendosulfan completely.
Materials and methods
Soil sampling
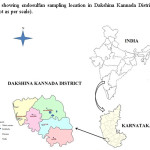
The soil samples were collected from cashew plantations of Kokkada, Patrame and Nidle villages in Belthangady Taluk of Dakshina Kannada District, Karnataka were the soils were exposed to endosulfan for decades.The soil samples were collected from different sites of the same field of cashew plantation (Fig.2.). The collected soil samples were kept in labeled polythene covers and brought to laboratory for experimental analysis. Then, the samples were air dried, sieved through a 2mm mesh and stored at 4â°C until further use. The basic soil characteristics are shown in the Table 1.
Chemicals
Analytical grade α- Endosulfan (99.6%) and β- Endosulfan (99.8%) were obtained from Sigma-Aldrich Co., USA and were used as standard. Stock solution (100 ppm) of each isomer was separately prepared in HPLC grade n-Hexane by weighing approximately 1 mg (Acculab ALC210.4) of the analyte into a 10 ml volumetric flask and diluting into a volume. Stock standard solutions were stored in the dark at -20â°C. Working solutions were prepared as and when required 26.
 |
|
Table 1: Basic soil characteristics of samples collected from endosulfan contaminated sites.
|
Village |
Soil collecting site |
Sample name
|
Carbon |
||||
|
Kokkoda |
Cashew plantation |
K-1 |
0.033 |
5.46 |
1.4 |
14.6 |
0.118 |
|
K-2 |
0.038 |
5.34 |
1.5 |
11.1 |
0.352 |
||
|
K-3 |
0.044 |
5.57 |
1.1 |
12.4 |
0.226 |
||
|
K-4 |
0.025 |
5.60 |
1.6 |
8.5 |
0.295 |
||
|
Patrame |
Cashew plantation |
P-1 |
0.015 |
5.84 |
1.1 |
10.1 |
0.132 |
|
P-2 |
0.023 |
4.50 |
1.8 |
11 |
0.155 |
||
|
P-3 |
0.061 |
4.45 |
1.2 |
9.1 |
0.183 |
||
|
Nidle |
Cashew plantation |
N-1 |
0.085 |
4.35 |
2.3 |
11.2 |
0.195 |
|
N-2 |
0.007 |
5.22 |
1.6 |
13.3 |
0.525 |
||
|
N-3 |
0.032 |
5.30 |
0.9 |
12.5 |
0.230 |
Enrichment and isolation of endosulfan degrading bacterial strain
For isolation, 1g of soil sample collected from endosulfan contaminated sites of cashew plantations of Dakshina Kannada District, was inoculated into 250ml Erlenmeyer flask containing 100 ml of Non sulfur Medium (NSM) of composition (g/l) K2HPO4 0.225g, KH2PO4 0.225g, NH4Cl 0.225g, MgCl2.6H2O 0.845g, CaCO3 0.005g, FeCl2.4H2O 0.005, D-glucose 1.0gm and 1ml trace element solution of (mg/l) MnCl2.4H2O 198mg, ZnCl2 136mg, CuCl2.2H2O 171mg, CoCl2.6H2O 24mg, NiCl2.6H2O 24mg [27] along with 100mg/l endosulfan as sole carbon source at pH 7.5, and kept for incubation at 120 rpm in shaker at 37â°C for 7 days. After 7 days of incubation, 1ml of sample was reinoculated into 100ml fresh media with 100 mg/lendosulfan. After fourth enrichment, the sample was spread plated on plates of mineral medium (1.5% agar) with 100mg/l endosulfan. The pure, potent strain isolated was characterized morphologically and biochemically and compared with Bergey’s manual of systematic bacteriology 28.
Biodegradation Studies
The pure potent strain showing luxury growth on NSM agar plate designated as ES-1 was selected for biodegradation of endosulfan isomers. For this, 250ml flask containing 100ml of NSM medium was inoculated with cell pellets of strain ES-1 (10 ml of overnight culture was taken in 15 ml centrifuge tube and was centrifuged at 5000 rpm for 10 min. Then, the supernatant was discarded and cell pellets was dissolved with 1 ml of sterile NSM medium and added into the flask aseptically) along with 70 mg/l of α isomer and 30 mg/l of β isomer and incubated at 37 The extraction of insecticide from the medium was carried out according to 29 with slight modification. After 7 days of incubation, 35ml of liquid media was taken in 50ml centrifuge tube. The tube was centrifuged at 10,000 rpm for 5min and supernatant was separated by separate flask using petroleum ether by shake flask method. Then, organic aqueous layer was passed through (~2 gm) anhydrous magnesium sulphate. Later the solution was concentrated by rotary evaporator (Bucchi type, GG Technologies, India). Similarly, after 14 days of incubation, the sample was extracted by same procedure. The residue was then dissolved in HPLC grade n-Hexane and was analyzed through Gas chromatography-mass spectroscopy (GC-MS).
Optimum Degradation studies
Optimum degradation condition for endosulfan by strain ES-1 was carried out according to 30 with slight modification. The degradation studies were carried out with different temperature (37 & 55â°C) and with different concentration of endosulfan (100, 250 & 500 mg/l).All the experiments were carried out in 250ml flasks containing NSM media with endosulfan as the sole carbon source. The pH of the medium was checked at the end of 14th day. The experiments were carried out in triplicates along with control. The extraction was carried out according to protocol mentioned above.
Microcosm studies on degradation of Endosulfan in soil
For soil microcosm studies, soil samples were collected from Mysore University campus, which didn’t had any application of pesticides previously. The collected samples were sieved by 2 mm mesh to remove debris. Then, 100 gm of soil samples were placed in 250 ml Erlenmyer flasks and autoclaved at 121â°C for 20 min. The procedure was repeated thrice so that, soil is free from all other microorganisms. Later, the soil samples were spiked with 100 mg kg-1 of endosulfan and inoculated with cell pellets containing 106 cells gm-1 of Bacillus sp. ES-1. Similarly control flasks were kept without the addition of cell culture. The flasks were kept for incubation for 7-14 days. The flasks were moistened at regular intervals by adding 10 ml of sterile water. The residues were then extracted according to 1 by shake flask method. 10 g of soil was taken in 250 ml Erlenmeyer flask and 50 ml of petroleum ether and acetone (1:1, v/v) was added and kept in horizontal shaker for overnight. Later, the extracts were filtered and kept for evaporation for dryness in a beaker. Later, the residues were dissolved in 1ml of HPLC grade n-hexane and were analyzed by GC-MS.
Residue analysis
GC-MS analysis was carried out on 7890A GC systems (Agilent Technologies, inc) linked to Mass spectroscopy of Synapt G2 HPMS MS (Waters, USA) equipped with Electron spray ionization (ESI) detector. The GC column consisted with HP-5MS capillary column of 30m x 0.250mm (Agilent Technologies, inc). The GC-MS was programmed according to.31The oven temperature was programmed to increase from 120â°C to 320â°C at 10â°C min-1. Mass spectra were recorded at 70 ev using full scan mode. The qualitative analysis of endosulfan present in the samples was monitored by comparing retention time with respect to internal standard. Recovery study was carried out to evaluate the efficiency of above said procedure. The average recovery for both endosulfan isomers was found to be 93% and 91.2% for α and β endosulfan respectively.
Identification of Strain ES-1 (DNA Isolation& 16S rDNA amplification)
DNA was isolated from the strain according to32The amplification of 16S rDNA gene of strain was carried out using polymerase chain reaction (PCR) according to33, by forward (BS F: GAGTTTGATCCTGGCTCA GG) and reverse (BS R: TCATCTGTCCCACCTTCGGC). The DNA sequences were analyzed with the Internet BLAST Gene database (http://www. ncbi.nlm.nih.gov) and the sequence was submitted to GenBank. Later, the partial sequenced data was submitted to Genbank and accession number KX230063 was obtained.
Results and Discussion
Isolation of endosulfan degrading bacterial isolate
The soil enrichment method adopted for isolating microbial culture resulted in isolation of bacterial strain which could tolerate high concentration of endosulfan. Out of three village samples, we were able to isolate bacteria from only Patrame village. After repeated successive sub-culturing in enrichment medium, microbial isolate designated as ES-1 showed substantial growth there by indicating the utilization of endosulfan as the growth substrate.
Identification of ES-1
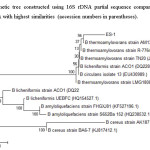
ES-1 was identified as gram-positive facultative anaerobic Bacilli shaped (Fig.3.), non-motile, Catalase positive, and exhibited creamish white colony morphology on NSM agar with endosulfan. The DNA isolated was subjected to PCR amplification. Based on 16s rDNA sequence the bacterium was identified as Bacillus sp. The Phylogenetic tree constructed using neighbor joining method using Mega software showed strainES-1 (KX230063) 99% query cover with Bacillus thermoamylovorans (KJ842641) (Fig.4.). However, other molecular evidence is to be studied further to conclude the strain ES-1 as Bacillus thermoamylovorans.
![Fig 3. Microscopic images of bacterial isolate ES-1 observed under Light Microscope (Allwin Scientifics), India [oil immersion (100X)]](http://www.cwejournal.org/wp-content/uploads/2017/08/Vol12_No1_Bio_sup_Fig3-150x150.jpg) |
|
Biodegradation of Endosulfan using Bacterial Isolate ES-1
Biodegradation of Endosulfan using bacterial isolate ES-1Degradation of endosulfan isomers (α & β) was assessed using bacterial strain ES-1.GC-MS analysis revealed that, after 14 days of incubation the strain ES-1 was able to metabolize endosulfan completely without formation of any known intermediates. The result is based on comparative analysis of total number of peaks obtained by GC-MS Chromatogram. , (Fig.5a.).Chromatograph ‘a’represents Total Ion Chromatograph of standard endosulfan with retention time of 11.21 for α-endosulfan and 11.74 for β-endosulfan with m/z 406 (Fig.5b.). Chromatograph ‘c’ (Fig.5c.) represents sample taken from flask after 14 days incubation with m/z 390.
 |
|
In Mineral medium with endosulfan as sulfur source, the organism capable of removing sulfite group and use it as sulfur source will survive and reproduce.30 It also results in reducing the toxicity of compound which helps in detoxification of the compound. Microbial degradation of endosulfan isomers by various bacterial and fungal isolates in aerobic condition have been studied vastly. Endosulfan sulfate, endosulfan diol, endosulfan ether, endosulfan hydroxyether, endosulfan lactone, endosulfan monoaldehyde and endosulfan dialdehyde have been reported as the major metabolites formed during the microbial degradation of endosulfan isomers.23,34Both these analyses strongly support the complete degradation of endosulfan by ES-1strain.
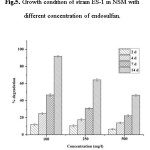
The Optimum biodegradation studies revealed that, the strain showed excellent growth at temperature 55â°C, than at 37â°C (Data not shown). The result revealed that, the Bacillus sp. ES-1 is a thermophilic bacteria surviving in acidic soil. The strain, ES-1 showed maximum degradation of >90% at 100 mg/l (Fig.5.) and was an efficient biodegrader of endosulfan provided at same concentration. The pH of the medium was reduced to acidic after 14 days of incubation (Table-2). The decrease in pH was observed more in 100 mg/l concentration than in 250 and 500 mg/l of endosulfan. The decrease in pH to acidic from neutral during endosulfan degradation was also observed by many other researchers.6, 35This is may be due to increased metabolic activity resulting in formation of acidic metabolites.
 |
|
The strain Bacillus sp. ES-1 followed hydrolytic pathway for biodegradation without formation of any known metabolites. These results revealed that the strain ES-1 adopted the hydrolytic pathway for endosulfan biodegradation,36 contrary to oxidative pathway of endosulfan biodegradation in which endosulfan sulfate is formed.37-39 The absence of any particular metabolite accumulation in the culture media during the course of degradation could suggest a possible complete mineralization of endosulfan through formation of some polar metabolites. Similarly, no metabolites were observed by during endosulfan degradation by Agrobacterium tumefaciens PT-3 suggesting possibility of utilizing insecticide as a carbon source by unique sets of genes and enzymes.20Achromobacterxyloxidans C8B was also able to mineralize isomers of endosulfan and Endosulfan sulfate provided at concentration of 50 ppm up to 20th day incubation in liquid media.30Complete mineralization of endosulfan by the bacterial strains Staphylococcus sp., Bacillus circulans I and II had been studied.These strains were found to be excellent degrader of endosulfan and its metabolites. The Mineralization of insecticide may harbor hydrolysis pathway resulting in formation of carbenium ions or ethyl carboxylates which will later convert into simple hydrocarbons.40
 |
|
Table 2: Decrease in pH was observed after 14 days of Incubation (Initial pH 7).
|
Sl.No. |
Pesticide Concentration (mg/l) |
pH after 14 days |
|
1 |
100 |
3.773 |
|
2 |
250 |
4.680 |
|
3 |
500 |
4.840 |
Biodegradation capability of Bacillus sp.ES-1 strain in soil for both α and β isomers (100 mg/kg) of endosulfan was assessed. The isolate was able to degrade 50% of compound in 7 days and complete mineralization was observed after 14 days. No accumulated product was detected.Whereas control flask extract showed both the isomers along with m/z of 423 corresponding to mass of endosulfan sulfate. This clearly explains that the strain followed hydrolytic pathway in mineralization of endosulfan. Under abiotic condition, the compound oxidized it into endosulfan sulfate. The previous studies have shown that, by hydrolysis both the isomers get hydrolyzed into endosulfan ether and diol27 and endosulfan sulfate through oxidation41. The mixed bacterial consortium was able to mineralize isomers of endosulfan without formation of any known intermediate metabolites40 contrast to this; endosulfan sulfate was formed by Bacillus sp.42.Microbial strains capable of removing insecticides without formation of toxic byproducts should be implemented in bioaugmentation or bioremediation than the strains forming toxic byproducts43. The results from present work revealed that, the isolate ES-1 hydrolyzed both the isomers of endosulfan completely without formation any toxic byproducts. Hence, it acts as an excellent bioaugmenting agent. the Bacillus sp ES-1 may harbor enzymes in degrading both the isomers which needs to be studied further.
Conclusion
Endosulfan isomers and endosulfan sulfate being toxic to humans and the environment, persists in soil for longer period. The indigenous bacterial strain Bacillus sp.ES-1 was found to degrade endosulfan completely. Endosulfan sulfate, an intermediate compound usually accumulates during the course of degradation, which is generally considered as more toxic and persistent than the parent compound was not detected. From these results, we conclude that, The indigenous strain ES-1 could be used as bioaugmenting agent to decontaminate the soil contaminated with high concentration of endosulfan.
Acknowledgment
The authors thank Department of Science and Technology (DST)-Science and Engineering Research Board (SERB), Government of India, for grant received (SB/EMEQ-041/2013) to carry out this work and Institution of Excellence (IOE), University of Mysore, Mysuru, for providing Gas Chromatography-Mass spectroscopy (GC-MS).
References
- Ozcan, S., Tor, A., Aydin, M. E. Analytical Methods for Viable and Rapid Determination of Organochlorine Pesticides in Water and Soil Samples, Pesticides - Strategies for Pesticides Analysis. InTech, 59-82 (2011)
- Unites States- Environmental Protection Agency (US-EPA),. Endosulfan RED facts. http://www.epa.gov/pesticides/registration/REDs/factsheet/endosulfan_fs.htm (2002) (Nov.6, 2014).
- Jennings, A. A., Li, Z. Residential surface soil guidance applied worldwide to the pesticides added to the Stockholm Convention in 2009 and 2011. Journal of Environmental Management,160(10):226-240(2015)
CrossRef - Schmidt, W. F., Bilboulian, S., Rice, C. P., Fettinger, J. C., McConnell, L. L., Hapemann, C. J. Thermodynamic, spectroscopic, and computational evidence for the irreversible conversion of β- to α-endosulfan. Journal of Agriculture and Food Chemistry,49(11):5372–5376(2001)
CrossRef - Agency for Toxic Substances and Diseases Registry (ASTDR) Draft Toxicology profile for Endosulfan. http://www.atsdr.cdc.goc/toxprofiles/tp41.pdf (2013) (Nov. 7, 2014).
- Siddique, T., Okeke, B. C., Arshad, M., Frankenberger, W. T. Enrichment and Isolation of Endosulfan-Degrading Microorganisms. Journal of Environmental Quality,32(1):47-54(2003)
CrossRef - Xiong, B. W. F., Zhou, A., Zheng, G., Zhang, J., Xu, W. Photocatalyic degradation of endosulfan in contaminated soil with the elution of surfactants. Journal of Soils and Sediments,15(9):1909-1918(2015)
CrossRef - Andreu, V., Pico, Y. Determination of pesticides and their degradation products in soil: critical review and comparison of methods. Trends in Analytical Chemistry,23(10-11):772–89(2004)
CrossRef - Jia, H., Sun, Y., Li, Y. F., Tian, C., Wang, D., Yang, M., Ding, Y., Ma, J. Endosulfan in China 2-emissions and residues. Environmental Science and Pollution Research,16(3): 302-311 (2009).
CrossRef - Jayashree, R., Vasudevan, N. Organochlorine pesticide residues in ground water of Thiruvallur District, India. Environmental Monitoring and Assessment,128(1):209-215(2007)
CrossRef - Zhao, Z., Zeng, H., Wu, J., Lu, Zhang. Organochlorine pesticide (OCP) residues in mountain soils from Tajikistan. Environmental Science: Processes & Impacts,15(3):608(2013)
CrossRef - Halse, A. K., Schlabach, M., Schuster, J. K., Jones, K. C., Steinnes, E., Breivik, K. Endosulfan, pentachlorobenzene and short-chain chlorinated paraffins in background soils from Western Europe. Environmental Pollution,196(1),21-28(2014)
- Mawussi, G., Scorza Junior, R. P., Dossa, E. L., AkoueteAlate, A. Insecticide residues in soil and water in coastal areas of vegetable production in Togo. Environmental Monitoring and Assessment,186(11):7379-7385(2014)
CrossRef - Odukkathil, G., Vasudevan, N. Residues of endosulfan in surface and subsurface agricultural soil and its bioremediation. Journal of Environmental Management,165(1):72-80(2016)
CrossRef - Arshad, M., Hussain, S., Saleem, M. Optimization of environmental parameters for biodegradation of alpha and beta endosulfan in soil slurry by Pseudomonas aeruginosa. Journal of Applied Microbiology,104(2):364-370(2007)
CrossRef - Kumar, M., Lakshmi, C. V., Khanna, S. Biodegradation and bioremediation of endosulfan contaminated soil. Bioresource Technology,99(8):3116-3122(2007)
CrossRef - Bajaj, A., Pathak, A., Mudiam, M. R., Mayilraj, S., Manickam, N. Isolation and characterization of Pseudomonas strain IITR01 capable of degrading α-endosulfan and endosulfan sulfate. Journal of Applied Microbiology,109(6):2135-2143(2010)
CrossRef - Kalyani, S. S., Sharma, J., Singh, S., Dureja, P., Lata. Enrichment and isolation of endosulfan-degrading microorganism from tropical soil. Journal of Environmental Science and Health Part B,44(7):663-672(2009)
CrossRef - Kumar, A., Bhoot, N., Soni, I., John, P. J. Isolation and characterization of a Bacillus subtilis strain that degrades endosulfan and endosulfan sulfate. 3 Biotech,4(5):467-475(2014)
- Thangadurai, P., Suresh, S. Biodegradation of endosulfan by soil bacterial cultures. International Biodeterioration & Biodegradation,94(4):38-47(2014)
CrossRef - Kumari, M., Ghosh, P., Swati., Thakur, I. S. Microcosmic study of endosulfan degradation by Paenibacillus ISTP10 and its toxicological evaluation using mammalian cell lines. International Biodeterioration & Biodegradation,96(7):33-40 (2014)
CrossRef - Singh, M., Singh, D. K. Biodegradation of Endosulfan in Broth Medium and in Soil Microcosm by Klebsiella M3. Bull Environ ContamToxicol,92(2):237-242(2014)
CrossRef - Abraham, J., Silambarasan, S. Biomineralization and formulation of endosulfan degrading bacterial and fungal consortiums. Pesticide Biochemistry and Physiology,116(6):24-31(2014)
CrossRef - Singh, D. K.Biodegradation and bioremediation of pesticide in soil: concept, method and recent developments. Indian J. Microbiol,48(1):35–40(2008)
CrossRef - Supreeth, M., Raju, N. S. Bio-mineralization of Organophosphorous Insecticide-Chlorpyrifos and its Hydrolyzed product 3,5,6-Trichloro-2- Pyridinol by Staphylococcus ES-2. Current World Environment,11(2):486-491(2016)
CrossRef - Murugan, A. V., Swarnam, T. P., Gnanasambandan, S. Status and effect of pesticide residues in soils under different land uses of Andaman Islands, India. Environmental Monitoring and Assessment,185(10):8135-8145(2013)
CrossRef - Hussain, S., Arshad, M., Saleem, M., Khalid, A. Biodegradation of α- and β-endosulfan by soil bacteria. Biodegradation,18(6):731-740(2007)
CrossRef - Vos, PD., Garrity, GM., Joned, D., Krieg, NR., Ludwig, W., Rainey FA., Schleifer, KH., Whitman WB. Bergey’s Manual of Systematic Bacteriology, 2nd edition, Vol.3. Springer (2009)
- Supreeth, M., Chandrashekar, M. A., Sachin, N., Raju, N. S. Effect of chlorpyrifos on soil microbial diversity and its biotransformation by StreptomycesHP-11. 3 Biotech, 6:147(2016)
- Singh, N. S., Singh, D. K. Biodegradation of endosulfan and endosulfan sulfate by Achromobacter xylosoxidans strain C8B in broth medium. Biodegradation,22(5):845-857(2011)
CrossRef - Kamei, I., Takagi, K., Kondo, R. Degradation of endosulfan and endosulfan sulfate by white-rot fungus Trametes hirsute. Journal of Wood Science,57:317-322 (2011)
CrossRef - Mora, D., Fortina, M. G., Parini, C., Daffonchio, D., Manachini, P. L. Genomic subpopulations within the species Pediococcus acidilactici detected by multilocus typing analysis: relationship between pediocin AcHPA-1 producing and non-producing strains. Microbiology, 146:2027–2038(2000)
CrossRef - Raghavendra, P., Halami, P. M. Screening, Selection and characterization of phytic acid degrading lactic acid bacteria from chicken intestine and their probiotic properties. Int J Food Microbiol,133(1-2):129–134(2009)
CrossRef - Rivero, A., Niell, S., Cesio, V., PiaCerdeiras, P., Heinzen, H. Analytical methodology for the study of endosulfan bioremediation under controlled conditions with white rot fungi. Journal of Chromatography B,907:168-172 (2012)
CrossRef - Bhalerao, T. S. Bioremediation of endosulfan-contaminated soil by using bioaugmentation treatment of fungal inoculants Aspergillus niger. Turk J Biol, 36:561-567(2012)
- Kim, Y. K., Kim, S. H., Choi, S. C. Kinetics of endosulfan degradation by Phanerochaete chroysosporium. Biotechnology letters,23(2):163-166(2001)
CrossRef - Kullman, S. W., Matsumura, F. Metabolic Pathways Utilized by Phanerochaete chrysosporium for Degradation of the Cyclodiene Pesticide Endosulfan. Applied and Environmental Microbiology,62(16):593-600(1996)
- Kwon, G. S., Shone, H., Shin, K. S., EungbinkimSeo, B. Biodegradation of the organochlorine insecticide, endosulfan and the toxic metabolite, endosulfan sulphate by Klebsiella oxymora KE-8. Appl Microbial Biotechnology,67(6):845-850 (2005)
CrossRef - Odukkathil, G., Vasudevan, N. Enhanced biodegradation of endosulfan and its major metabolite endosulfate by a biosurfactant producing bacterium. Journal of Environmental Science and Health, Part B,48(6):462-469(2013)
CrossRef - Kumar, M., Philip, L. Bioremediation of endosulfan contaminated soil and water-Optimization of operating conditions in laboratory scale reactors. Journal of Hazardous Materials B,136(2):354-364(2006)
CrossRef - Kwon, G. S., Kim, J. E., Kim, T. K., Sohn, H. Y., Koh, S. C., Shin, K. S., Kim, D. G. Klebsiella pneumonia KE-1 degrades endosulfan without formation of the toxic metabolite, endosulfan sulfate. FEMS MicrobiolLett,215(2):225-259(2002)
CrossRef - Shivaramaiah, H. M., Kennedy, I. R. Biodegradation of Endosulfan by a Soil Bacterium. Journal of Environmental Science and Health, Part B,41(6):895-905(2006)
CrossRef - Supreeth, M., Raju, N. S. Biotransformation of chlorpyrifos and endosulfan by bacteria and fungi. Applied Microbiology and Biotechnology, 101(15):5961-5971(2017)
CrossRef






