Bio-Mineralization of Organophosphorous Insecticide-Chlorpyrifos and its Hydrolyzed Product 3,5,6-Trichloro-2-Pyridinol by Staphylococcus Sp. ES-2
M. Supreeth 1 * and N. S. Raju 1
DOI: http://dx.doi.org/10.12944/CWE.11.2.17
Application of Chlorpyrifos on agricultural fields to protect crops against pests results in accumulation of it in soil and other environmental samples. The insecticide transform into 3,5,6-Trichloro-2-Pyridinol (TCP) through hydrolysis in soil, which has got antimicrobial property and hence resists its degradation in natural condition. In the current findings, a bacterial isolate capable of mineralizing Chlorpyrifos without accumulation of TCP was isolated from agricultural soil by enrichment method. Based on Morphological, Biochemical Characterization and with Bergey’s Manual comparision, the isolate was identified as Staphylococcus sp. The isolate was found to metabolize chlorpyrifos completely in Mineral salt medium with chlorpyrifos as the sole carbon source. No metabolites of chlorpyrifos were detected in Liquid Chromatography-Mass Spectroscopy (LC-MS) analysis after 7 days of incubation. The novelty of the outcome of the experiment relies on Staphylococcus sp.ES-2 in complete mineralization of chlorpyrifos which can be used as a potential bioaugmenting agent in the chlorpyrifos contaminated sites.
Copy the following to cite this article:
Supreeth M, Raju N. S. Bio-Mineralization of Organophosphorous Insecticide-Chlorpyrifos and its Hydrolyzed Product 3,5,6-Trichloro-2-Pyridinol by Staphylococcus Sp. ES-2. Curr World Environ 2016;11(2) DOI:http://dx.doi.org/10.12944/CWE.11.2.17
Copy the following to cite this URL:
Supreeth M, Raju N. S. Bio-Mineralization of Organophosphorous Insecticide-Chlorpyrifos and its Hydrolyzed Product 3,5,6-Trichloro-2-Pyridinol by Staphylococcus Sp. ES-2. Curr World Environ 2016;11(2). Available from: http://www.cwejournal.org/?p=14198
Download article (pdf)
Citation Manager
Publish History
Select type of program for download
| Endnote EndNote format (Mac & Win) | |
| Reference Manager Ris format (Win only) | |
| Procite Ris format (Win only) | |
| Medlars Format | |
| RefWorks Format RefWorks format (Mac & Win) | |
| BibTex Format BibTex format (Mac & Win) |
Article Publishing History
| Received: | 2016-04-18 |
|---|---|
| Accepted: | 2016-05-13 |
Introduction
Large quantities of pesticides are used in agriculture throughout the world and most of them are toxic to both humans and animals. Once the pesticide is applied in agricultural field, it remains in the soil for longer periods and sometimes it gets transformed into various byproducts. These byproducts may impact on Environment
Organophosphorus (OPPs) pesticides are beeing widely used in agricultural practice for more than 40 years. The toxic accumulation of OPPs is achieved by inhibiting acetylcholinesterase, an enzyme necessary for normal nerve impulse transmission. Most of the OPPs have similar general structure, containing three phosphoester linkages, and hydrolysis of one of the phosphor ester bonds dramatically reduce the toxicity of the pesticides by eliminating their acetylcholinesterase-inactivating properties.1
OPPs accounts Ë‚34% or world’s total pesticide application for controlling pests of various crops in different countries. Application of OPPs in the fields or accidental release leads to contamination of surface and ground water. These compounds are being highly mammalian toxicity, has to be removed from the environment.2
Chlorpyrifos(CP) O, O-diethyl O-(3,5,6 – trichloro -2n pyridyl phosphorothioate) is a crystalline organophosphate insecticide. It was discovered by Dow chemical company in 1965. CP hydrolyses rapidly in soil into its primary metabolite 3,5,6 trichloro-2-pyridinol (TCP)3 and this TCP has antimicrobial property, which prevents the degradation of CP by soil microorganisms.4 The studies on the fate of TCP and its degradation in environment are very much limited
CP is the second most detected insecticide in food and water because of its broad usage in agriculture on various crops. This has raised public concern demand to remove it from the contaminated sites.5
Removal of CP in Environment through biotic degradation is one of the most viable option. Several researchers have focused on microbial degradation of CP.6-14
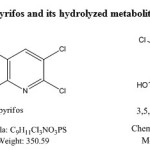 |
|
Despite several papers reporting biodegradation of chlorpyrifos by microbial cultures, only few isolates have capable of degrading both chlorpyrifos and its hydrolysis product 3,5,6 trichloro-2-pyridinol. In this context, we framed objective to isolate and degrade both these compounds by enrichment technique.
Materials and Methods
Sample Collection and Chemicals
The soil samples were collected from sugar cane and rice field area of Hosahalli, Haniyambadi village, Mandya Taluk, Karnataka. The collected soil samples were air dried, sieved through a 2mm mesh and stored at 4â° C until further use. Analytical grade chlorpyrifos was obtained from Sigma-Aldrich Co., USA. Stock solution (100 ppm) of CP was prepared in HPLC grade Acetonitrile by weighing approximately 1mg of compound into a 10ml volumetric flask and diluting into a volume. Stock standard solutions were stored in the dark at -20â°C. Working solutions were prepared as and when required.15
Enrichment and Isolation of Chlorpyrifos Degrading Bacterial Strain
The bacterial strain was screened from soil samples by enrichment technique. For isolation, 1g of soil sample was inoculated into 250ml Erlenmeyer flask containing 100 ml of MSM medium (Table-1) and 100 mg L-1 chlorpyrifos as sole carbon source at pH 7 and kept for incubation at 120 rpm in shaker at 37â°C for 7 days. After 7 days of incubation, 35mL culture aliquot was taken in 50ml centrifuge tube and centrifuged at 10,000 rpm for 5min. later the supernatant was taken in separate flask and extracted using equal volume of acetonitrile by shake flask method and the organic aqueous layer was separated. The solvent was evaporated by rotary evaporator. The residues was then dissolved in HPLC grade acetonitrile and analyzed by TLC followed by LC-MS.
Table: 1: Composition of MSM Medium In 1000 ml distilled water, pH=7.6
|
Ingredients |
Quantity |
|
Dipotassium hydrogen phosphate(K2HPO4) |
1.5 g |
|
Monopotassium phosphate (KH2PO4) |
0.5 g |
|
Ammonium sulphate (NH4)2SO4 |
0.5 g |
|
Sodium chloride (Nacl) |
0.5 g |
|
Magnesium Sulphate (MgSO4) |
0.2 g |
|
Calcium chloride (CaCl2) |
0.05 g |
|
Ferrous sulphate (FeSO4) |
0.02 g |
Identification of the Isolated Chlorpyrifos Degrading Bacterial Strain
The potent strain isolated was characterized Morphologically and Biochemically and compared with Bergey’s manual of systematic bacteriology.
LC-MS Analysis
After 07 days of incubation, 35mL culture aliquot was taken in 50ml centrifuge tube and centrifuged at 10,000 rpm for 5min. later the supernatant was taken in separatory flask and extracted using equal volume of acetonitrile by shake flask method and the organic aqueous layer was separated. The solvent was evaporated by rotary evaporator. The residue was then dissolved in HPLC grade acetonitrile and analyzed using liquid chromatography-mass spectroscopy (LC-MS) (Acquity Waters, USA)according to.16 The LC-MS was equipped with a BEHC 181.7µm column (10 x 50mm) with auto injector. The cartridges were conditioned with acetonitrile and washed with deionized water containing 0.1 % formic acid. Mass spectroscopy (MS) was performed using a Synapt G2 HPMS MS (Waters, USA) equipped with Electron spray ionization (ESI) detector. The operating condition was Capillary (kV)-3.00, Sampling Cone-40.00, Extraction Cone-4.00, Source Temperature (â°C)-100, Desolvation Temperature (â°C)-200, and Desolvation Gas flow (L/Hr)-500.0.
Results and Discussions
Isolation of Chlorpyrifos Degrading Bacteria
In the present study, selective enrichment method was used to isolate chlorpyrifos-degrading bacteria from paddy field. Eight soil samples were screened for isolating potent bacterial strain. From eight samples, three distinct strains were obtained, and among which bacterial strain designated ES-2 was chosen for analysis due to its highest tolerance to chlorpyrifos (100 mg L-1) as the sole carbon source on Mineral salt agar medium plates.
Identification of ES-2
The bacterial strain ES-2 was identified as gram-positive facultatively anaerobic coocci. The bacterial strain is non-motile, Catalase positive, oxidasde negative and exhibited creamish white colony morphology. According to the Bergey’s Manual of Systematic Bacteriology, the bacterium comes under order Bacillales, family Staphylococcaceae and genus Staphylococcus. Biochemical test results (Table-1) show the close relatedness of strain ES-2 with genus Staphylococcus.
Table 2: Biochemical tests results
|
Test |
Results |
|
Catalase |
+ |
|
Urease |
+ |
|
Starch hydrolysis |
- |
|
Mannitol salt agar |
+ |
|
Glucose |
+ |
|
Lactose |
- |
|
Sucrose |
- |
Biodegradation Ability of Staphylococcus sp. ES-2
The biodegradation of chlorpyrifos (100 mg L-1) was assessed using Staphylococcus sp ES-2. Thin Layer Chromatography (TLC) followed by LC-MS was used to monitor the disappearance of chlorpyrifos and TCP. The result of TLC analyses revealed the formation of unknown metabolites. The LC-MS analysis showed that the bacteria was able to efficiently degrade chlorpyrifos without formation of TCP (based on m/z value obtained). The current findings suggest that the isolate used this compound as carbon and energy source for its growth by forming polar metabolites. In most of the research outcomes on biodegradation of CP, the microorganisms hydrolysis it into TCP, which accumulates in the culture medium or soil and avoids further degradation of the same because of its antimicrobial property.17-19 Environmental Protection Agency (EPA) of USA has found, TCP has been associated with estrogenic activity and has been listed as potential endocrine disrupting chemical.
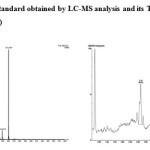 |
|
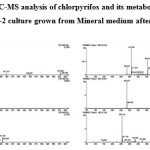 |
|
Pseudomonas putida was reported to metabolize Chlorpyrifos after the strain immobilized with calcium alginate, the products of degradation detected by LC-MS was found to be TCP and Chlorpyrifos oxon.19 TCP was accumulated during the degradation process of 100 mg L-1 of Chlorpyrifos by Sphingomonas sp., which slowed down the degradation process.19
The Mechanism of chlorpyrifos degradation in bacteria is fairly understood and a number of degradation products such as diethylthiophosphoric acid, TCP, Chlorodihydro-2-pyridone and Maleamide semialdehyde have been identified.21 In the present study, it was found that the bacterial isolate ES-2 was able to degrade chlorpyrifos with complete mineralization. The occurrence of unidentified peaks in chromatogram of present study may be byproducts of one of the above said products, which need further investigation.
Conclusions
Bioremediation, biodegradation and bioaugmentation based techniques are gaining popularity for the purpose of ecological restoration. Potential indigenous microbial strain, isolated from the area where contamination has occurred over a years can be used as successful bioaugmenting agent to remove toxic contaminants. These indigenous microbe give duel benefit, first they detoxify the contaminant and secondly do not pose any serious threat to other native flora and fauna. Many biotic and abiotic factors significantly influence the process of biodegradation. Results from the present study confirm that the isolated chlorpyrifos-degrading bacterium could be successfully implemented for removal of chlorpyrifos contaminated sites.
Acknowledgment
The authors thank Department of Science and Technology (DST)-Science and Engineering Research Board (SERB), Government of India, for grant received to carry out this work and Institution of Excellence (IOE), University of Mysore, Mysuru, for providing Liquid Chromatography-Mass spectroscopy (LC-MS).
References
- Horne, I., Sutherland, T. D., Harcourt, R. L., Russell, R. J., & Oakeshott, J. G., Identification of an opd (organophosphate degradation) gene in an Agrobacterium Appl Environ Microbiol, 68: 3371–3376 (2002).
- Sing, B. K., & Walker, A., Microbial degradation of organophosphorus compounds. FEMS Microbiol Rev, 30: 428–471 (2006).
CrossRef - Racke, K. D., Steele, K. P., Yoder, R. N., Dick, W. A., & Avidov, E., Factors affecting the hydrolytic degradation of chlropyrifos in soil. Agric. Food Chem. 44: 1582–1592 (1996).
CrossRef - Li, J., Liu, J., Shen, W., Zhao, X., Hou, Y., Cao, H., & Cui, , Isolation and characterization of 3,5,6-trichloro-2-pyridinol-degrading Ralstonia sp. strain T6. Bioresour Technol, 101:7479–83 (2010).
CrossRef - John, E. M., Shaike, J. M., Chlorpyrifos: pollution and remediation. Chem. Lett, 13(3), 269–291 (2015).
CrossRef - Sasikala, C., Jiwal, S., Rout, P., & Ramya, M., Biodegradation of chlorpyrifos by bacterial consortium isolated from agriculture soil. World Journal of Microbiology and Biotechnology , 28: 1301-1308 (2012).
CrossRef - Lakshmi, C. V., Kumar, M., Khanna, S., Biodegradation of chlorpyrifos in soil by enriched cultures, Curr Microbiol, 58: 35–38 (2009).
CrossRef - Das, S., & Adhya, T. K., Degradation of chlorpyrifos in tropical rice soils. J Environ Manage, 152: 36–42 (2015).
CrossRef - Ghanem, I., Orfi, M., & Shamma, M., Biodegradation of Chlorpyrifos by Klebsiella isolated from an activated sludge sample of waste water treatment plant in Damascus. Folia Microbiol, 52: 423–427 (2007).
CrossRef - Zhu, J., Zhao, Y., Qiu, J., Isolation and application of a chlorpyrifos-degrading Bacillus licheniformis ZHU-1. J. Microbiol. Res, 4: 2410-2413 (2010).
- Singh, D. P., Khattar, J. I. S., Nadda, J., Singh, Y., Garg, A., Kaur, N., & Gulati, A., Chlorpyrifos degradation by the Cyanobacterium Synechocystis strain PUPCCC 64. Environ Sci Pollut Res, 18:1351–1359 (2011).
- Chishti, Z., Hussain, S., Arshad, K. R., Khalid, K., Arshad, A., Microbial degradation of chlorpyrifos in liquid media and soil. J Environ Manage, 114:372–380 (2013).
- Briceno, G., Fuentes, M. S., Palm, G., Jorquera, M. A., Amoroso, M. J., & Diez, M. C., Chlorpyrifos biodegradation and 3,5,6-trichloro-2-pyridinol production by Actinobacteria isolated from soil. International Biodeterioration & Biodegradation 73, 1-7 (2012).
- Barathidasan, K., Reetha, D., John Milton, D., Sriram, N., & Govindammal, M., Biodegradation of chlorpyrifos by coculture of Cellulomonas fimi and Phanerochaete chrysosporium. Afr J Microbiol Res, 8(9): 961–966 (2014).
CrossRef - Murugan, A. V., Swarnam, T. P., Gnanasambandan, S., Status and effect of pesticide residues in soils under different land uses of Andaman Islands, India. Environ Monit Assess, 185:8135–8145(2013).
CrossRef - Harishankar, M. K., Sasikala, C., & Ramya, M., Efficiency of the intestinal bacteria in the degradation of the toxic pesticide, chlorpyrifos.3Biotech, 137–142 (2013).
- Anwar, S., Liaquat, F., Khan, Q. M., Khalid, Z. M., & Iqbal, S., Biodegradation of chlorpyrifos and its hydrolysis product 3,5,6-trichloro-2-pyridinol by Bacillus pumilus strain C2A1 Journal of Hazardous Materials, 168: 400–405 (2009).
CrossRef - Racke, K. D., Laskowsky, D. A., Shultz, M. R., Resistance of chlorpyrifos to enhanced biodegradation in soil. Agric. Food Chem, 38: 1430-1436 (1990).
CrossRef - Xu, G., Zheng, W., Li, Y., Wang, S., & Zhang, J., Biodegradation of chlorpyrifos and 3,5,6-trichloro-2-pyridinol by a newly isolated Paracoccus sp. strain TRP, Int Biodeterior Biodegradation, 6: 51–56 (2008).
CrossRef - Pradeep, V.,& Subbaiah, U. M., Repeated batch and continuous degradation of chlorpyrifos by Pseudomonas putida. J Environ Sci Health B, 50: 346-360 (2015).
CrossRef - Li, X., Jiang, J., Gu, L., Ali, S. W., He, J., Li, S., Diversity of chlorpyrifos-degrading bacteria isolated from chlorpyrifos-contaminated samples. Int Biodet Biodegrad, 62:331–5 (2008)
CrossRef - Sing, B. K., & Walker, A., Microbial degradation of organophosphorus compounds FEMS Microbiol Rev, 30:428–471 (2006).
CrossRef






